Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Antibiotic prophylaxis is recommended during initial treatment of ANCA-associated vasculitis (AAV). We assessed characteristics associated with prophylaxis within a Canadian multicenter AAV cohort starting rituximab (RTX) for either induction or maintenance of remission.
Methods: We performed a secondary analysis of baseline characteristics from the Biosimilars in ANCA-associated Vasculitis compared to the Originator (BRAVO) cohort, including adults with granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA) who started RTX induction or maintenance (either the originator or a biosimilar) at cohort entry. We stratified characteristics (at time of starting RTX induction or maintenance, as applicable) according to concurrent antibiotic prophylaxis (e.g. trimethoprim sulfamethoxazole [TMP-SMX], dapsone, atovaquone), and compared groups using 95% confidence interval (CI) for the differences in mean or proportion, or the Mann Whitney U test as applicable. Characteristics of interest included demographics, current prednisone dose, disease activity, disease duration and prior relapse. Univariable and multivariable logistic regressions assessed the association of covariates of interest with prophylaxis.
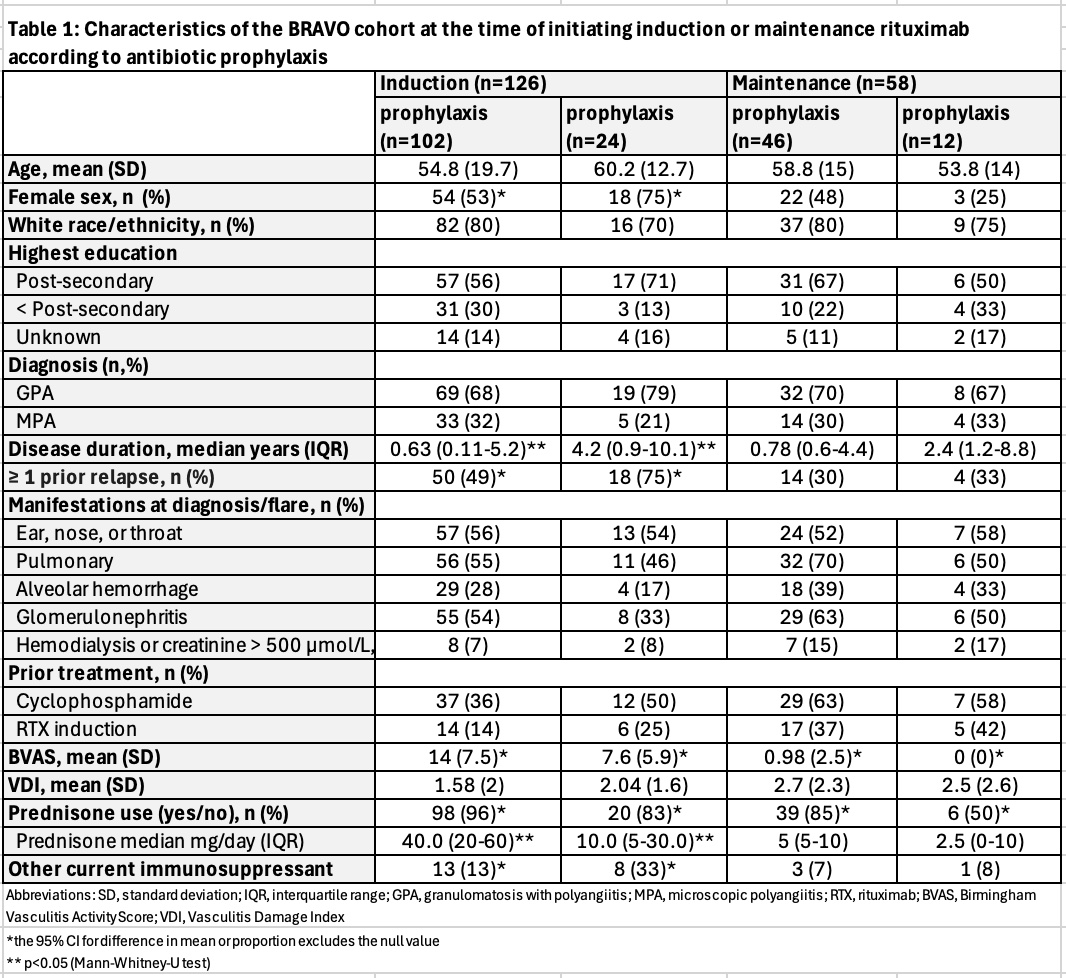
Results: Of the 200 participants in BRAVO, 126 initiated induction and 58 initiated maintenance at baseline. [Table 1]. The proportion taking prophylaxis at the start of induction (81%) and maintenance (79%) was similar, whereas 98 (96%) of the induction group and 39 (85%) of the maintenance group were receiving prednisone at baseline. TMP-SMX was the most commonly used antibiotic during induction (95%) and maintenance (89%), with the remainder being primarily atovaquone (2% induction, 11% maintenance) and dapsone (2% in induction).In the induction group, any prednisone use (OR 4.9; 95% CI 1.1-21.4), prednisone ≥20 mg/day (OR 6.5; CI 2.5-17.0) and baseline Birmingham Vasculitis Activity Score (BVAS) (OR for each point 1.2; 95 CI 1.1-1.3) were associated with antibiotic prophylaxis, while use of other concurrent immunosuppressants (OR 0.3; 95% CI 0.1-0.8) and having ≥1 prior relapse (0.3 OR; 95% CI 0.1-0.9) were negatively associated with prophylaxis. In multivariable analyses, daily prednisone dose ≥20 mg (adjusted OR 3.4; 95% CI 1.2-10.0) and BVAS (aOR 1.1, 95% 1.0-1.2) remained associated with prophylaxis. In the maintenance group, prednisone use was also associated with prophylaxis in adjusted analyses (aOR 6.1, 95% CI 1.3-27.0).
Conclusion: In this multicenter cohort initiating RTX for induction or maintenance, 80% of the participants were taking antibiotic prophylaxis, which is higher than reported in other cohorts. During the induction and maintenance period, concurrent prednisone was associated with prophylaxis, as was higher disease activity during induction. Our findings provide additional insight into the patterns of antibiotic prophylaxis use in AAV.
To cite this abstract in AMA style:
Le Blanc N, Barra L, Bernatsky S, Clifford A, Dabaghjamanesh M, Dehghan N, Fifi-Mah A, Makhzoum J, Milman N, Soowamber M, Khalidi N, Pagnoux C, Meunier R, Cohen Tervaert J, Yacyshyn E, Mendel A. Antibiotic Prophylaxis during Treatment of ANCA-associated Vasculitis with Rituximab: Data from a Multicenter Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/antibiotic-prophylaxis-during-treatment-of-anca-associated-vasculitis-with-rituximab-data-from-a-multicenter-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibiotic-prophylaxis-during-treatment-of-anca-associated-vasculitis-with-rituximab-data-from-a-multicenter-cohort/

.jpg)
.jpg)