Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To evaluate the safety and effectiveness of rituximab biosimilars in the treatment of small vessel vasculitis.
Methods: The Medline, CINAHL, Cochrane, Embase and Web of Science databases were searched from inception until May 7, 2025 to identify all studies (full-length manuscripts or abstracts) including ³5 adults diagnosed with any small vessel vasculitis treated with a rituximab biosimilar for induction or maintenance of remission, including switching from the originator to a biosimilar. Effectiveness outcomes were clinical remission (Birmingham Vasculitis Activity Score = 0) and relapse, while serious adverse events, serious infections, hypersensitivity reactions, late-onset neutropenia and hypogammaglobulinemia were safety outcomes.
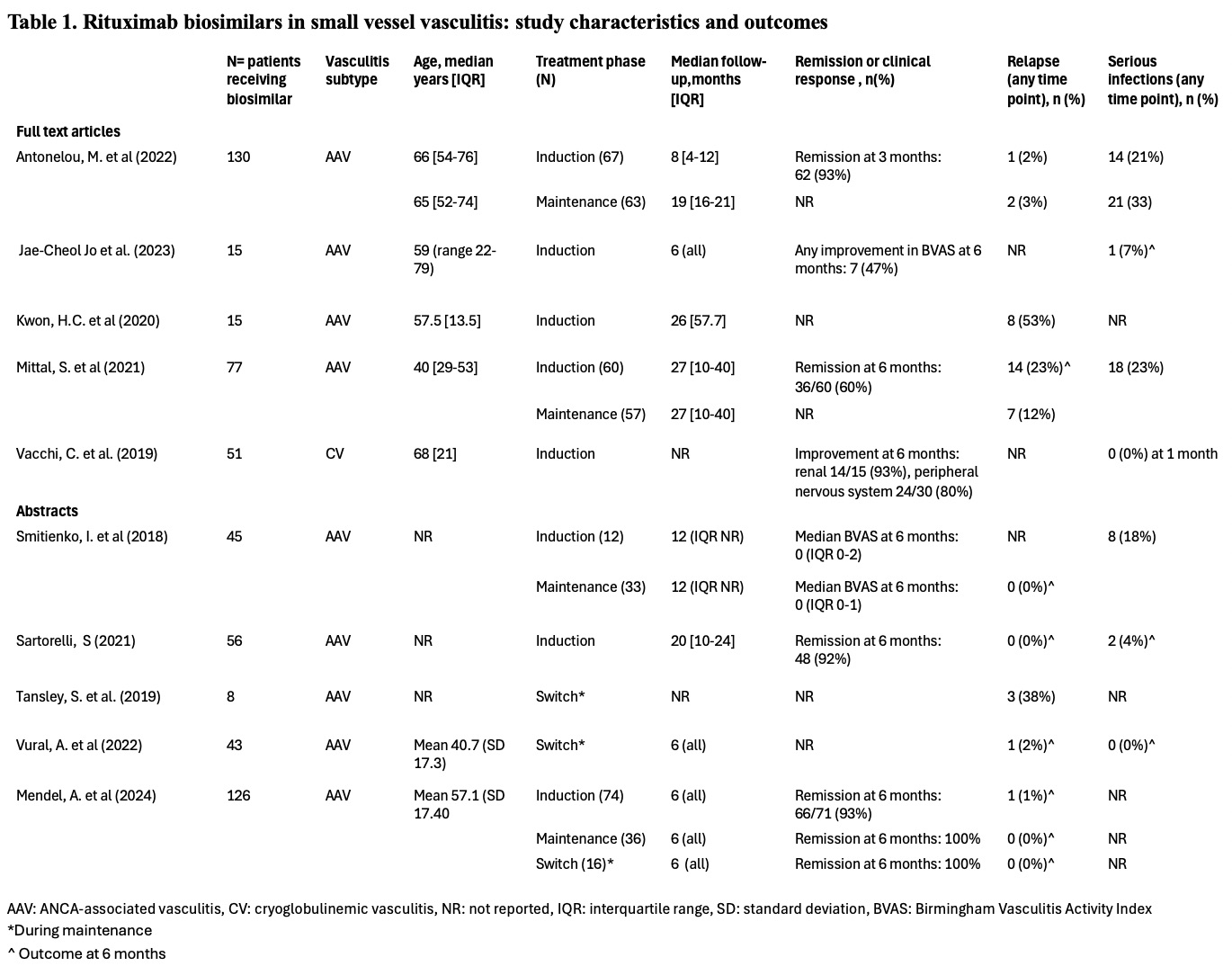
Results: Of 1,628 publications screened, 69 underwent full text review, and ten (5 full text articles, 5 abstracts) reporting on 566 patients treated with rituximab biosimilars were included (n=9 ANCA-associated vasculitis [AAV], n=1 cryoglobulinemic vasculitis [CV]). Seven studies were retrospective, while three were prospective. Most studies reported on the biosimilar Truxima (n=6), while Ruxience, Acelbia and Reditux were evaluated in one study each, and biosimilar type was not indicated in one abstract. Due to heterogeneous outcome reporting, we did not perform a meta-analysis. Follow-up ranged from 5-27 months after first rituximab biosimilar dose. Among studies evaluating a rituximab biosimilar for AAV induction (n=7), clinical remission at 6 months ranged from 60-93%, and relapses occurred in 0-23% of patients by 6 months (reported in 3 studies each). Among studies assessing a rituximab biosimilar for AAV maintenance (n=6), relapses occurred in 0-2% by 6 months (three studies). Of 3 studies evaluating outcomes after switching from originator to biosimilar in stable AAV, two reported sustained remission in 98-100% of patients at 6 months, while one observed relapses in 3/8 patients (timeframe undefined). In the CV study, renal parameters improved in 14/15 (93%) patients and peripheral neuropathy improved in 24/30 (80%) at 6 months. Three studies reported ≥1 serious infection in 0-7% of patients by 6 months. Five studies reported hypersensitivity reactions (2-14%). Hypogammaglobulinemia occurred in 4% and 31% of patients, and late-onset neutropenia in 2% and13% of patients (reported in two studies each). Four studies compared a rituximab biosimilar to the originator (3 AAV, 1 CV), with no significant differences found in terms of remission, relapse, serious adverse events, or infections.
Conclusion: We did not identify any randomized studies of biosimilar rituximab for small-vessel vasculitis, and observational data is limited. The biosimilars studied in AAV appear to have safety and effectiveness similar to historical randomized controlled trials and observational studies of the originator molecule.
To cite this abstract in AMA style:
Kanters C, Lee J, Pagnoux C, Barra L, Makhzoum J, Mendel A. Effectiveness and Safety of Rituximab Biosimilars in Small Vessel Vasculitis: a Systematic Literature Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-rituximab-biosimilars-in-small-vessel-vasculitis-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-rituximab-biosimilars-in-small-vessel-vasculitis-a-systematic-literature-review/