Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: For the treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), rituximab (RTX) may be given as a 4-dose regimen (375 mg/m2 weekly for 4 weeks) or a 2-dose regimen (1000 mg on day 0 and 14, D0-D14) to induce remission. Once the remission is achieved, RTX is administered in the maintenance phase (starting 4 to 6 months after the first induction dose) at 500 mg every 6 months (Q6M) for 18 months or at 1000 mg every 4 months (Q4M) for 20 months. In the absence of direct comparison of these dosing regimens, there is a need to better define the optimal regimen as well as the duration of the maintenance phase in order to improve the risk-benefit ratio while reducing treatment costs. We aimed to provide pharmacokinetic-pharmacodynamic (PK/PD) rationale for the selection of optimal induction and maintenance dosing regimens in AAV using a population modeling approach based on longitudinal data (RTX concentrations, MPO-ANCA, PR3-ANCA and gammaglobulins) from a real-world cohort.
Methods: A total of 121 patients with 296 plasma RTX concentrations (99 and 197 in the induction and maintenance phases, respectively), 439 ANCA levels (215 in the induction, 224 in the maintenance) and 559 gammaglobulin levels (249 in the induction, 310 in the maintenance) were included in the analysis. The modeling of the effect of plasma RTX concentrations on gammaglobulin and ANCA levels over time was preformed using non-linear mixed effects models in Monolix Suite. Simulations of induction (375 mg/m2 weekly for 4 weeks, 1000 mg D0-D14) and maintenance regimens (500 mg every 6 months [Q6M], 500 mg Q6M starting at month 4, 1000 mg Q4M, 500 mg Q4M) were performed in 1000 virtual patients with the final PK/PD model. Dosing regimens were compared based on the percentage of patients in serological remission and at risk of hypogammaglobulinemia. In addition, a utility score was calculated taking into account both the expected gain in efficacy and the increased risk of adverse events with increased drug exposure.
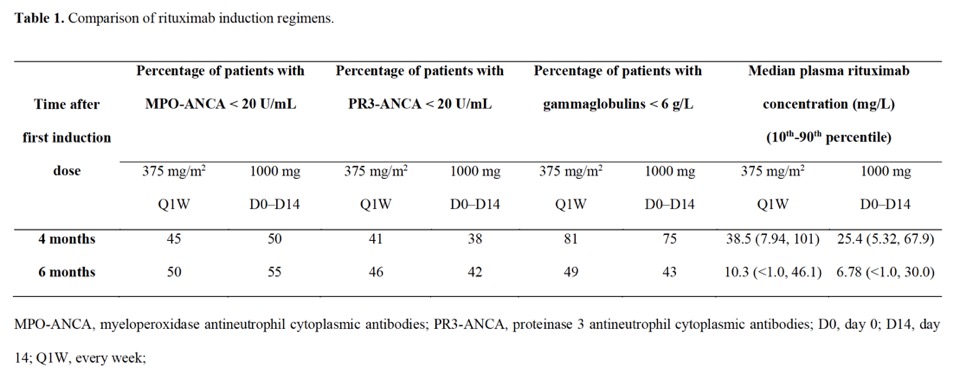
Results: The final PK/PD model satisfactorily described the relationship between RTX concentrations, gammaglobulins and ANCA levels over time. Simulations showed that both induction regimens resulted in a similar number of patients achieving serological remission and hypogammaglobulinemia at 6 months (Table 1). In the maintenance phase, increasing the dose (1000 mg) or frequency of administration (Q4M versus Q6M) was associated with a higher number of patients with serological remission at month 24 but also with higher risk of hypogammaglobulinemia (Table 2). Starting the maintenance phase earlier, i.e. 500 mg Q6M from month 4 versus from month 6, was associated with a higher number of patients with negative MPO-ANCA and PR3-ANCA at month 24 (68% versus 59% for MPO-ANCA and 58% versus 46% for PR3-ANCA, respectively). Based on the benefit-risk ratio, both 500 mg Q6M and 500 mg Q4M regimens appear to be optimal for maintenance therapy.
Conclusion: This first PK/PD study may inform clinical decisions regarding the choice between different rituximab induction and maintenance regimens in AAV patients in daily practice.
To cite this abstract in AMA style:
Pasquiers B, Blanchet B, Puéchal X, Declèves X, Cohen P, Goulvestre C, Casadevall M, Benhabiles I, Vidal M, Ternant D, Terrier B, Puszkiel A. Comparison of rituximab induction and maintenance regimens in ANCA-associated vasculitis: PK/PD modelling approach in real-world patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparison-of-rituximab-induction-and-maintenance-regimens-in-anca-associated-vasculitis-pk-pd-modelling-approach-in-real-world-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-rituximab-induction-and-maintenance-regimens-in-anca-associated-vasculitis-pk-pd-modelling-approach-in-real-world-patients/

.jpg)