Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The Definition Of Remission In SLE (DORIS) criteria were developed to provide alignment on defining remission in patients with SLE.1,2 Belimumab (BEL) treatment increases the proportion of patients with SLE in DORIS remission.3 Several real-world studies have evaluated DORIS remission in BEL-treated patients with SLE4,5,6; however, data are limited for patients in the USA.
Methods: This retrospective, observational cohort study (GSK Study 222161) assessed remission among adult patients with SLE receiving BEL in a real-world setting in the USA using the OM1 PremiOM SLE dataset (OM1, Inc, Boston, MA, USA). Study period: Jan 1, 2013, to May 31, 2024. Eligible patients had ≥1 BEL pharmacy/medical encounter during the identification period (Jan 1, 2014, [index: first BEL receipt during identification period] to Oct 31, 2023). Primary objective: determine the proportion of patients achieving SLE remission (DORIS definition by proxy: SLEDAI score=0 [no disease activity]; Physician’s Global Assessment [PGA] < 2; prednisone equivalent dose ≤5 mg/day) at 28, 48, and 52 weeks (wks) post–BEL initiation. Secondary objectives included: describe SLEDAI scores and PGA before BEL and 28, 48, and 52 wks post–BEL initiation. Two censoring approaches assessed estimate robustness (last available score: earlier of (1) latest available SLEDAI or PGA score in follow-up period and (2) end of follow-up; end of follow-up: earlier of 52 wks post-index, last activity date, or date of data cutoff). Analyses were descriptive.
Results: Overall, 398 patients were included (Table 1). Estimates were sensitive to censoring approach (Table 2). By last available score, point estimates for the proportion of patients achieving SLE remission (per DORIS proxy) increased from 7.7% at 28 wks to 25.9% at 48 wks and 28.3% at 52 wks post–BEL initiation. The proportion of patients achieving no disease activity (SLEDAI=0) within 28 wks was 21.3%, increasing to 45.8% within 52 wks post–BEL initiation. The proportion of patients achieving PGA-based remission thresholds increased from 45.9% at 28 wks to 68.5% at 52 wks. By end of follow-up approach, the proportion of patients achieving SLE remission increased from 5.0% at 28 wks to 13.0% at 48 wks and 13.8% at 52 wks post–BEL initiation. Point estimates for the proportion of patients achieving no disease activity were 18.6% within 28 wks post–BEL initiation increasing to 29.5% within 52 wks. The proportion of patients achieving PGA-based remission thresholds was 43.7% at 28 wks and 56.3% at 52 wks.
Conclusion: This study demonstrates the achievement of real-world remission targets for patients with SLE initiating BEL in the USA, with DORIS remission criteria being reached within 28 wks for about 5–8% of patients, and within 52 weeks for 14–28% of patients.Funding: GSKReferences1van Vollenhoven R et al. Ann Rheum Dis 2017;76:554–61 2van Vollenhoven RF et al. Lupus Sci Med 2021;8:e0005383Parodis I et al. Lancet Rheumatol 2024;6(11):e751–614Nikoloudaki M et al. Front Immunol 2023;13:10740445Su Z et al. Arthritis Res Ther 2024;26:1636Altabás-González I et al. Rheumatology 2025;64:276–82Original presentation: CCR-East 2025
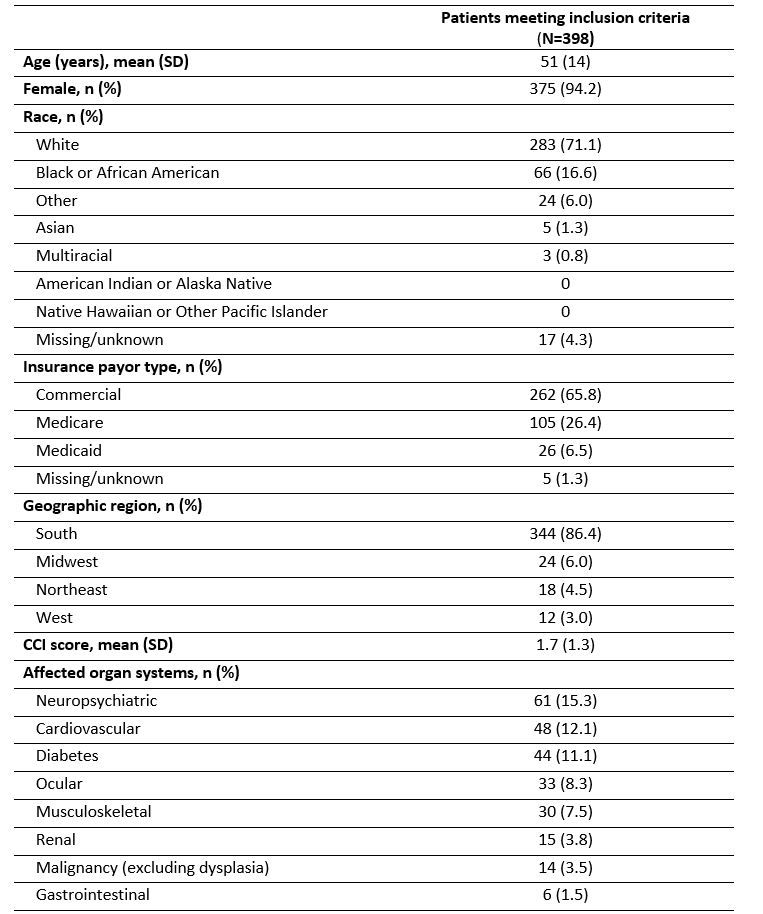 Table 1. Baseline demographics and clinical characteristics.*
Table 1. Baseline demographics and clinical characteristics.*
*The baseline period was defined as the period that begins 12 months prior to the index date up to and excluding the index date.
CCI, Charlson Comorbidity Index; SD, standard deviation.
.jpg) Table 2. Proportion of patients achieving real-world SLE remission (per DORIS proxy), no disease activity (per SLEDAI), and PGA-based threshold for remission.
Table 2. Proportion of patients achieving real-world SLE remission (per DORIS proxy), no disease activity (per SLEDAI), and PGA-based threshold for remission.
*The two censoring approaches were utilized to assess the robustness of estimates: the last available score which was the earlier of latest available PGA or SLEDAI score in the follow-up period (not carried forward) and end of follow-up, and the end of follow-up which was the earlier of 52 weeks post-index, last activity date or date of data cutoff; †the proportion of patients achieving SLE remission (per DORIS proxy) at 28, 48, and 52 weeks after initiating therapy with belimumab defined as SLEDAI score=0, PGA score < 2, and prednisone-equivalent dose ≤5 mg/day; ‡the proportion of patients with no disease activity (per SLEDAI; SLEDAI=0); §the proportion of patients with at least one PGA score meeting the predefined threshold for remission ( < 2).
CI, confidence interval.
To cite this abstract in AMA style:
Patel A, Gennarelli R, Bello T, Bonakdar A, Worley K. Evaluating Systemic Lupus Erythematosus Remission Among Patients Initiating Belimumab in a Real-World Setting in the USA [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluating-systemic-lupus-erythematosus-remission-among-patients-initiating-belimumab-in-a-real-world-setting-in-the-usa/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-systemic-lupus-erythematosus-remission-among-patients-initiating-belimumab-in-a-real-world-setting-in-the-usa/
