Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Emerging therapies such as CD19 CAR-T-cells have been reported to induce deeper B-cell depletion and thereby drug-free remission up to 18 months. With rituximab, we showed that efficacy is related to depth of depletion and rate of repopulation, which are highly variable, and related to FCGR genotype[1]. We have observed “RTX-super-responders” with sustained remission after one cycle of RTX. The objectives of this study were to assess a) incidence of RTX-super-response, b) factors associated with sustained remission.
Methods: We conducted an observational study of RTX-treated SLE patients in a single centre over 20 years with on-demand retreatment . Usual practice was to continue concomitant immunosuppressants but taper glucocorticoids. Univariable and multivariable logistic regression analyses were performed to identify factors associated with RTX long-response, with p < 0.1 associated with the deviance used for inclusion into the model.
Results: Of 149 first-cycle-RTX-treated patients, 114 were included in the study [excluded due to non-response in Cycle 1=17; received fixed retreatment at 6-9 months=15; deaths within the first 3 years=2; and discontinued RTX due to psoriasis=1]. Based on survival curve, we defined super-responders as >3 years. This occurred in 23/114 patients (20%) with median (IQR) duration of response 263 (212,423) weeks.At baseline, RTX-super-responders had: mean (SD) age 35 (14) years, 20/23 (87%) female, ancestry European=9/23 (39%); South Asian=6/23 (26%), Chinese/SE Asian=2/23 (9%); African=5/23 (22%); and Mixed=1/23 (4%], concurrent APS 6/23 (26%), disease duration 2.8 (1,5) years, median (IQR) SLEDAI-2K 11 (7-15), and median (IQR) numeric BILAG score 21 (13-25). Sustained suppression of plasmablasts was observed at 3 years: median (IQR) 0.0008 (0.0002-0.0045). 8/23 RTX-super-responders (34.8%) were either not prescribed immunosuppressants or withdrew them during follow up (drug free remission).In multivariable analysis of all 114 patients, RTX-super-response was associated with non-European ancestry (OR 4.6, 95% CI 1.6-12.7)) and concurrent APS, (3.2, 0.99-10.35). Longer disease duration (0.89,0.80-0.99 per year) was associated with lower odds of RTX-super-response. No other factors (age, sex, anti-dsDNA+, low C3/C4, number of antibodies, concomitant immunosuppressant, disease activity score, and active BILAG A/B in 5 most frequent domains) were predictive.
Conclusion: Sustained drug-free remission is not confined to patients who have received CAR-T CD19. 1 in 5 RTX-treated patients had >3 years response to their first cycle, and 1 in 12 had sustained, immunosuppressant-free remission. RTX-super-responders have patient characteristics denoting disease severity (early disease, non-European ancestry and APS), and marked suppression of plasmablast repopulation. This suggests that sustained drug-free remission is a feature of the overall immune environment rather than the modality of B cell killing. Future work will include more detailed biomarker evaluation of this cohort.
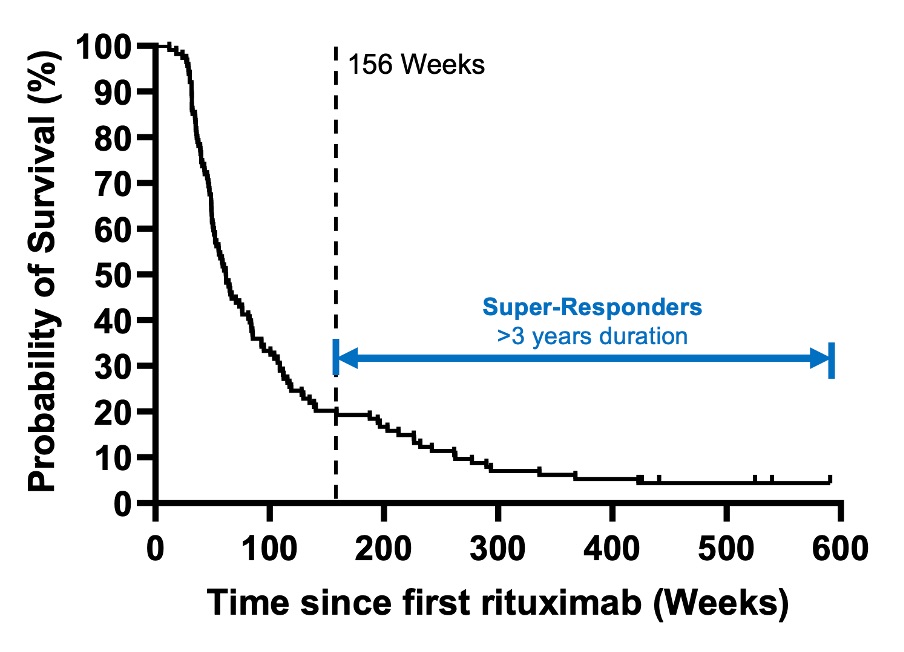 Relapse-free survival after first cycle of rituximab
Relapse-free survival after first cycle of rituximab
.jpg) Disease activity and plasmablast repopulation
Disease activity and plasmablast repopulation
To cite this abstract in AMA style:
Md Yusof M, Patel J, Emery P, Vital E. Rituximab super-responders: characteristics of patients with more than 3 years response to a single cycle of treatment [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rituximab-super-responders-characteristics-of-patients-with-more-than-3-years-response-to-a-single-cycle-of-treatment/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-super-responders-characteristics-of-patients-with-more-than-3-years-response-to-a-single-cycle-of-treatment/
