Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Biologic treatments for lupus nephritis (LN) have improved short-term outcomes. Obinutuzumab (OBI), an anti-CD20 monoclonal antibody, demonstrated efficacy in the NOBILITY and REGENCY trials; however, long-term real-world data are limited.
Methods: We conducted a retrospective single-center study of 26 LN patients (pts) who received at least 1g of OBI between 2015 and 2025. Clinical and laboratory data were collected to assess longitudinal changes in serum creatinine, eGFR, urine protein-to-creatinine ratio (UPCR), and B cells. A mixed-effects model was used to analyze changes in eGFR and UPCR over 48 months. Three pts with progressive renal decline either initiated hemodialysis or were lost to follow-up. B cell reconstitution kinetics, LN flare rates, and adverse events (AE) were analyzed. Renal responses were classified as complete, partial, or no response. Complete (CR) and partial remission (PR) were defined as follows: CR: UPCR < 0.5 and eGFR ≥85% of baseline; PR: ≥50% reduction in UPCR from baseline, UPCR < 1 (or < 3 if baseline was ≥3), and eGFR ≥85% of baseline.
Results: Of 26 pts who received OBI, 25 with adequate follow-up were analyzed (Table 1). UPCR levels decreased significantly over time (p < 0.001). There was no statistically significant difference in eGFR across time points. By 24 months, CR was achieved in 10 pts (40%), PR in 8 (32%), and NR in 7 pts (28%) (Fig. 1). Most CR and PR responses occurred between 6-18 months. Of 6 pts who flared, 2 CR pts flared 5 years after receipt of OBI, and 4 PR pts flared within 1 year. Nine pts in the CR and PR groups were able to discontinue steroids. Among the 7 pts who previously received rituximab, 3 did not respond to OBI. Across response groups, baseline UPCR values were similar: CR: 4.5; PR: 5.5; NR: 4.7 (p = 0.78). Pts achieving CR had a significantly higher baseline mean eGFR compared to NR (102 vs 58 mL/min/1.73 m²; p = 0.014). The mean difference in baseline eGFR between PR (80) and NR (58) was not significant (p = 0.32). Over 24 months, 50% of CR pts demonstrated stable or improved eGFR (mean change: +36%), and 50% had a decrease in eGFR (mean change: –7%). In the PR group, 83% of pts had stable or improved eGFR (mean change: +19%), and 17% had a decrease. When combined (CR + PR), 62% of pts had stable or improved eGFR (mean change +31%), while 38% had a decline in eGFR (mean change -6%). No significant association was found between baseline LN class and response outcome. Peripheral B cell counts decreased from a median of 48 cells/μL (IQR 12–121) at baseline to 3 cells/μL (IQR 1–39) at ~6 months. Reconstitution began by 12 months (median 14, IQR 0–55) and continued through 24 months (median 41, IQR 5–87). Of 12 pts with B cell data through 24 months, a total of 8, 11, and 12 repleted ( >10 cells/ μL) at 12, 18, and 24 months, respectively. AEs occurred in 18 pts, mostly mild or moderate. Infections and infusion-related reactions were the most common, with most events occurring within 6 months (Table 2).
Conclusion: Despite inadequate responses to prior therapies, two-thirds of our cohort achieved CR or PR. AEs were generally mild. These findings support the potential role of OBI as a long-term therapeutic option in LN.
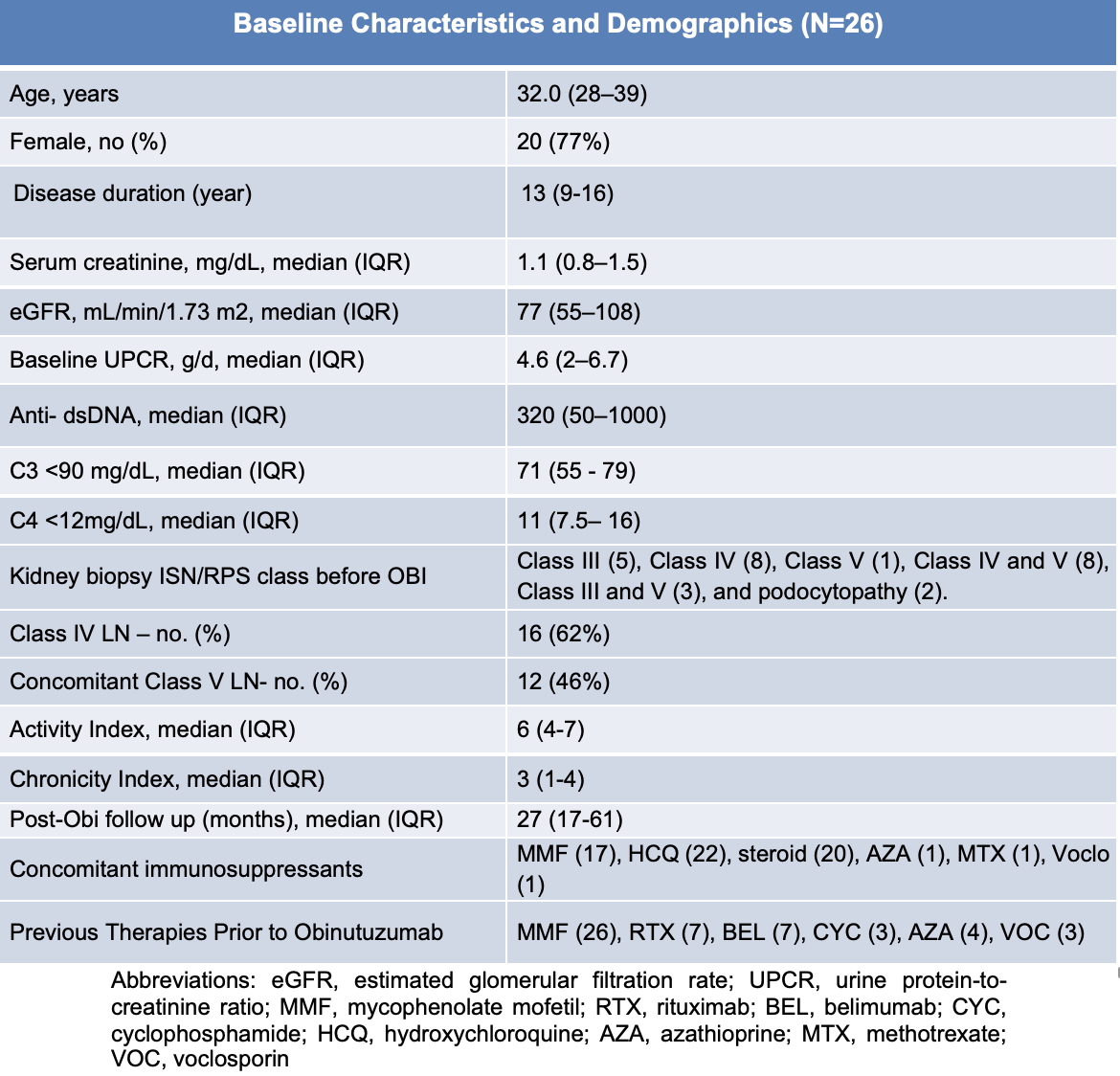 Table 1: Baseline Demographics and Clinical Characteristics
Table 1: Baseline Demographics and Clinical Characteristics
.jpg) Table 2: Adverse Events Associated with Obinutuzumab
Table 2: Adverse Events Associated with Obinutuzumab
.jpg) Fig 1. Longitudinal Treatment Responses in Patients Receiving Obinutuzumab Between 2015 and 2025
Fig 1. Longitudinal Treatment Responses in Patients Receiving Obinutuzumab Between 2015 and 2025
To cite this abstract in AMA style:
Toz B, Vashistha H, Furie R. Long-Term Effects of Obinutuzumab on Kidney Function in Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-effects-of-obinutuzumab-on-kidney-function-in-lupus-nephritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-effects-of-obinutuzumab-on-kidney-function-in-lupus-nephritis/
