Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab, a monoclonal antibody targeting the type I interferon receptor, demonstrated clinical efficacy in systemic lupus erythematosus in the pivotal TULIP-2 trial¹. However, its role in discoid lupus erythematosus (DLE) remains less well defined. Emerging case-based studies indicate potential benefit in refractory DLE². This review aims to evaluate the clinical role of anifrolumab in DLE using a focused literature review and meta-analysis.
Methods: To evaluate the clinical role of anifrolumab in DLE, we conducted a comprehensive literature search of MEDLINE and EMBASE for studies published between January 2015 and April 2025. We prioritized studies that reported Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity (CLASI-A) and – Damage (CLASI-D) scores to ensure a standardized assessment of treatment response. We included 8 studies (n = 36) for CLASI-A and 5 studies (n = 25) for CLASI-D. A random-effects meta-analysis using the DerSimonian-Laird method was applied. In studies lacking reported variability, we estimated standard deviations as 25% of the mean, consistent with methods used in prior meta-analyses of small clinical samples. All patients had received hydroxychloroquine and at least one prior immunosuppressive therapy.
Results: The meta-analysis demonstrated a significant reduction in CLASI-A scores among 36 patients with available data, with a pooled mean difference of –16.41 (95% CI: –19.72 to –13.10; p < 0.001; I² = 97.9%), indicating meaningful improvement in disease activity. For CLASI-D, assessed in 25 patients, the pooled mean difference was –0.63 (95% CI: –2.33 to 1.06; p= 0.465; I² = 78.2%), suggesting no statistically significant change in cutaneous damage. Among the 89 patients included across all studies, only 36 had CLASI-A scores and 25 had CLASI-D scores reported. Most patients also exhibited features of systemic lupus, including arthritis, serologic abnormalities, or other systemic involvement. Although the remaining cases lacked formal CLASI documentation, most were described as having improvement in their skin disease based on physician assessment or clinical descriptors. Average CLASI-A scores dropped from approximately 14.7 at baseline to 2.0 after treatment, while CLASI-D decreased from 4.9 to 3.0. No hospitalizations or deaths were reported. Six patients developed mucocutaneous herpes simplex virus (HSV) or varicella-zoster virus (VZV) infections, including one case of herpes zoster oticus. Among 22 patients with corticosteroid data, the average prednisone dose was reduced from 13.75 mg/day to 2.5 mg/day following anifrolumab treatment.
Conclusion: Anifrolumab appears effective and well tolerated in DLE, particularly in reducing disease activity and corticosteroid burden. Its impact on reversing cutaneous damage is limited. These findings support its role as a targeted therapy for DLE. While promising, the results are based on a small sample size and retrospective data, underscoring the need for validation in larger, prospective studies.References:Furie R et al. N Engl J Med. 2020;382(3):211-221.Shaw K et al. Arthritis Rheumatol. 2023;75(Suppl 9).
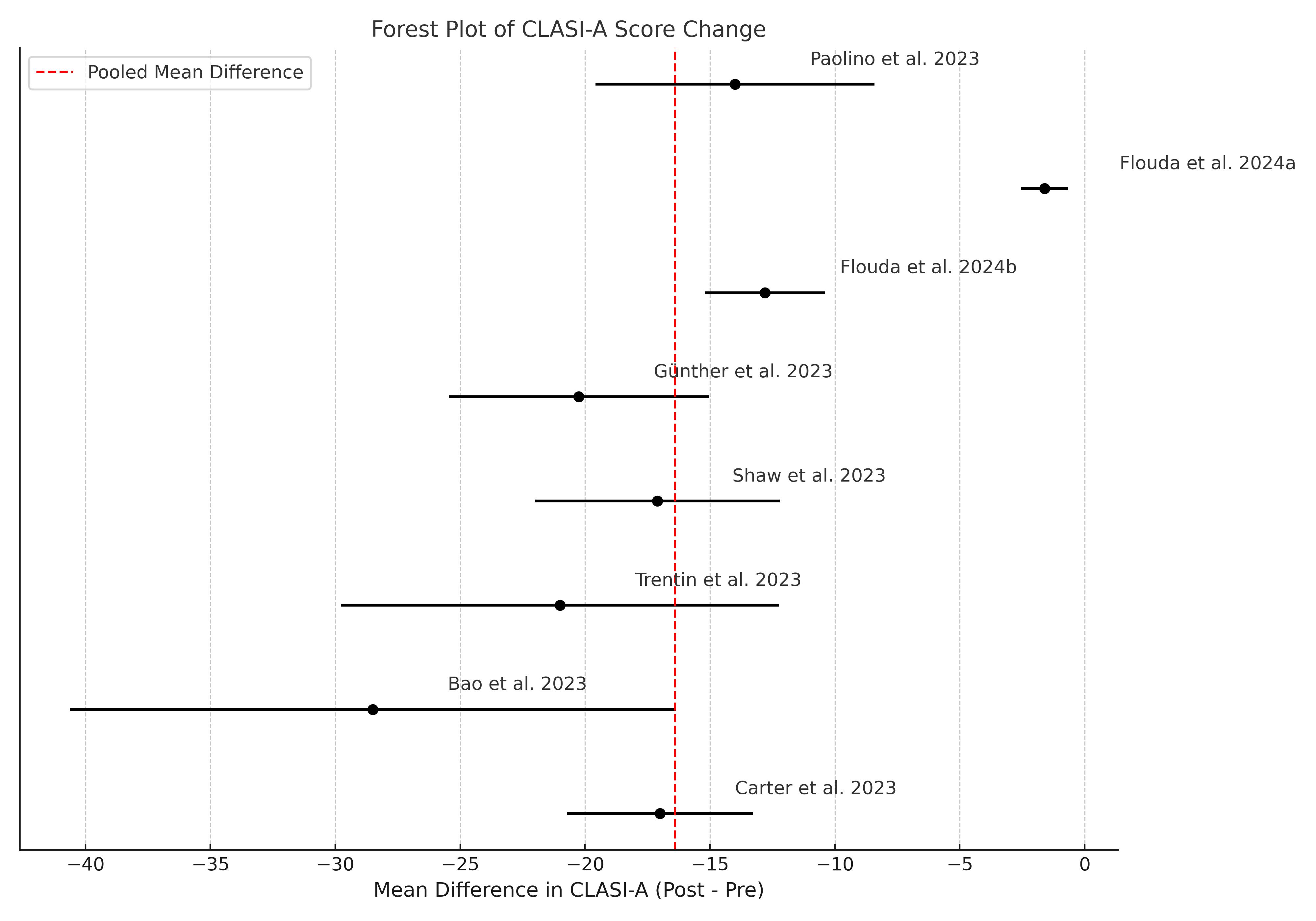 Forest Plot of CLASI-A Score Change
Forest Plot of CLASI-A Score Change
.jpg) Forest Plot of CLASI-D Score Change
Forest Plot of CLASI-D Score Change
To cite this abstract in AMA style:
Ali A, Purohit N, Robinson C, Darzi A, Meysami A. Efficacy and Safety of Anifrolumab in Discoid Lupus: A Meta-Analysis of the Literature [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-anifrolumab-in-discoid-lupus-a-meta-analysis-of-the-literature/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-anifrolumab-in-discoid-lupus-a-meta-analysis-of-the-literature/
