Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Nonadherence to medication is a significant confounder in assessing treatment efficacy for systemic lupus erythematosus (SLE), with rates reported up to 75% depending on the measurement method. Mycophenolate mofetil (MMF) presents unique adherence challenges due to twice-daily dosing, large pill size, and gastrointestinal side effects. Measuring serum mycophenolic acid (MPA: active metabolite of MMF) levels have been used to gauge compliance. However, MPA levels are costly, unavailable during patient encounters, and only performed by a few labs. In this study, we aimed to determine the most reliable predictor of adherence to MMF in SLE patients using multiple modalities. Barriers to compliance were also explored.
Methods: Adult patients with SLE on stable MMF dosing for at least six weeks followed at the outpatient clinic under the NIH longitudinal cohort study, were included. Patients with significant renal dysfunction (eGFR < 30 mL/min/1.73m²) or hypoalbuminemia (albumin < 3.5 g/dL) were excluded. Adherence was assessed using four methods: (1) Serum MPA trough levels were measured after an overnight fast and prior to the morning dose, with MPA < 1 mcg/mL considered nonadherent; (2) Pharmacy refill percentage was calculated as the proportion of MMF doses dispensed over the prescribed interval, with ≥80% considered adherent; (3) Patient self-assessment was quantified using modified Medication Adherence Self-Report Inventory (MASRI), with adherence defined as ≥80% by visual analog scale; and (4) Treating clinicians, blinded to MASRI scores and refill data, rated adherence using a 0–10 Likert scale. Patients were asked to provide reasons for non-compliance. Demographic and clinical data were collected via chart review and standardized survey. Comparisons between adherent and nonadherent groups were performed using t-tests and chi-square analyses, and correlations between adherence measures were assessed using Pearson coefficients.
Results: Of the 61 patients on stable MMF, 44 (72.1%) were classified as adherent based on MPA trough levels > 1mcg/ml. Pharmacy refill percentage was the only variable significantly associated with adherence: adherent patients had a higher mean refill percentage (0.89 ± 0.11) compared to nonadherent patients (0.66 ± 0.33; p There was a trend toward higher adherence among married patients (56.8% vs. 29.4%, p=0.10). Age, BMI, number of comorbidities, total number of medications, and MMF dosage were similar between groups. No significant differences were observed in ethnicity, sex, education, side effects, or MMF intake with food (Table 2). Forgetfulness and medication access were the most commonly identified barriers (Figure 1).
Conclusion: Only pharmacy refill percentage reliably predicted MMF adherence. Compliance can be improved by providing social support and using a reliable medication reminder system.
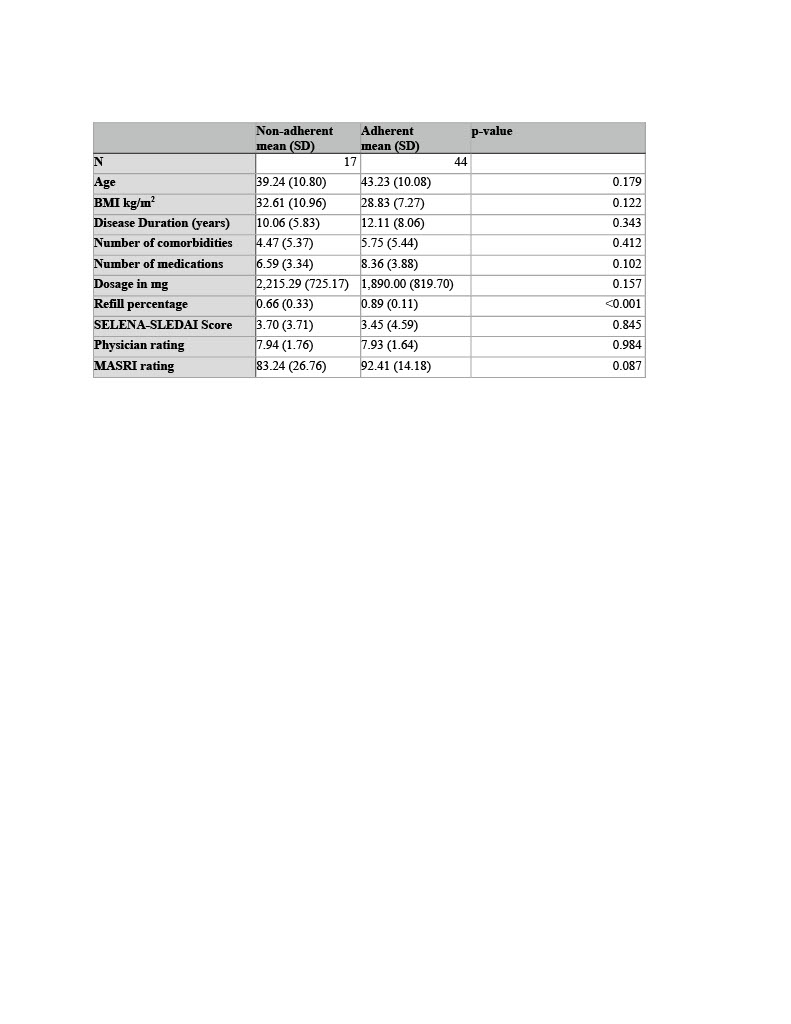 Table 1. Measures of MMF adherence
Table 1. Measures of MMF adherence
.jpg) Table 2. Demographic and Miscellaneous variables
Table 2. Demographic and Miscellaneous variables
.jpg) Figure 1. Word Cloud generated from patient responses. Reasons given for nonadherence
Figure 1. Word Cloud generated from patient responses. Reasons given for nonadherence
To cite this abstract in AMA style:
Bukhari N, Rizvi A, sun J, Manna Z, Schaughency P, Rajasimhan S, Hasni S. Why Do Some Lupus Patients Skip Their Mycophenolate Mofetil? A Multi-Factorial Assessment of Patient Compliance [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/why-do-some-lupus-patients-skip-their-mycophenolate-mofetil-a-multi-factorial-assessment-of-patient-compliance/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/why-do-some-lupus-patients-skip-their-mycophenolate-mofetil-a-multi-factorial-assessment-of-patient-compliance/
