Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment with the oral JAK inhibitor upadacitinib (UPA) has shown efficacy and safety in patients with active axial spondyloarthritis (axSpA), including both radiographic (r-axSpA, historically known as ankylosing spondylitis [AS]) and non-radiographic (nr-axSpA). This therapeutic benefit was observed among patients who are naïve to biologic DMARDs (bDMARD-naïve) as well as those with an intolerance or inadequate response to biologic DMARDs (bDMARD-IR). Sustainability of response was previously demonstrated through week 52 among patients who achieved clinical responses at week 14.1 This post hoc analysis evaluated the long-term sustainability of clinical outcomes through 2 years (104 weeks) in UPA-treated patients with axSpA from the SELECT-AXIS 1 and SELECT-AXIS 2 clinical trials.
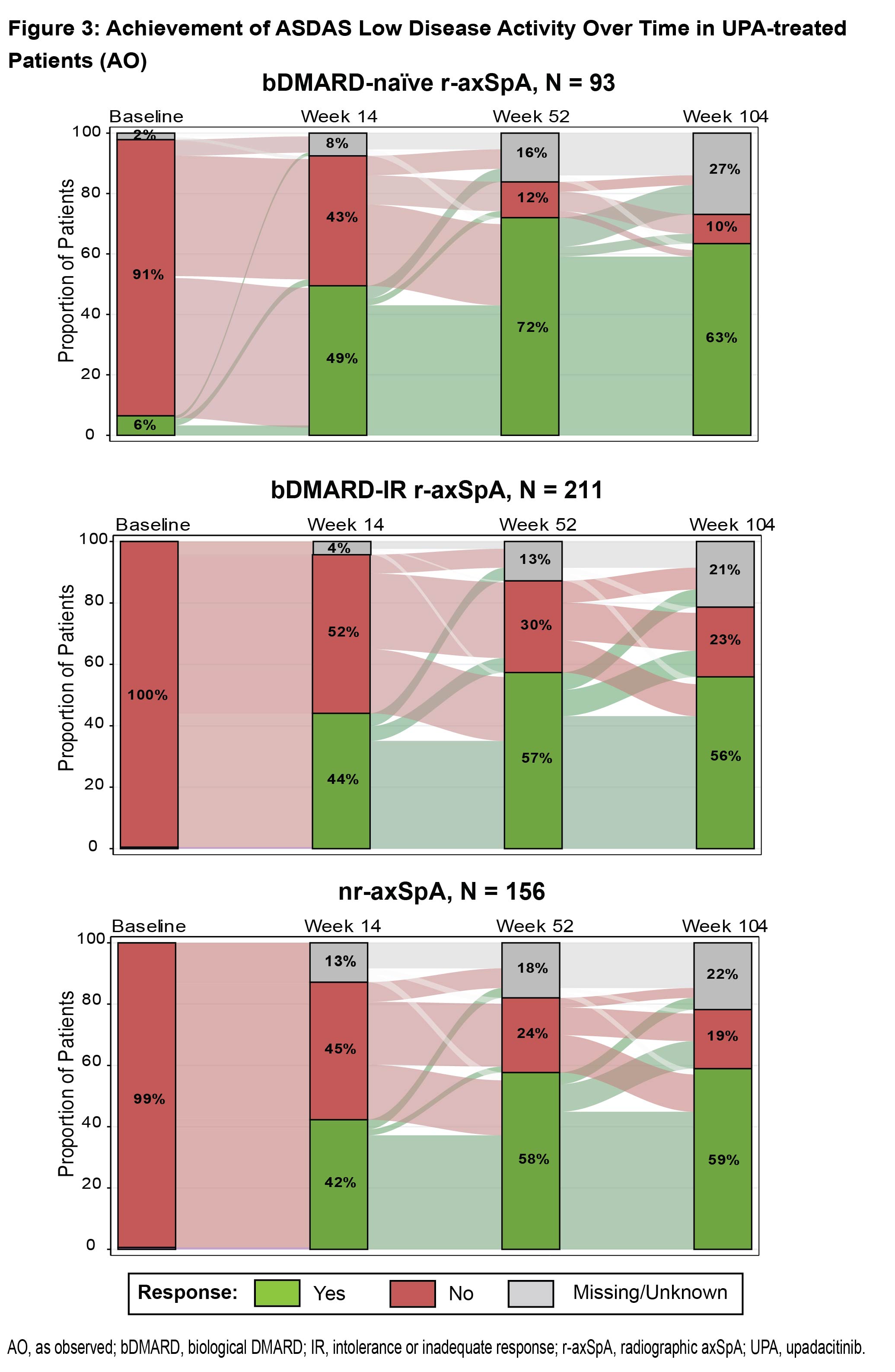
Methods: Data from SELECT-AXIS 1 (bDMARD-naïve r-axSpA) and SELECT-AXIS 2 (two studies: bDMARD-IR r-axSpA and nr-axSpA which included a population with 33% bDMARD-IR and 67% bDMARD-naïve) were assessed in this post hoc analysis. Sustained treatment response at week 104 was evaluated in patients receiving UPA 15 mg once daily who achieved responses at both weeks 14 and 52. Endpoints included ASAS40 response, ASDAS low disease activity (LDA; score < 2.1), and achievement of a minimal clinically important difference (MCID) in total and nocturnal back pain (≥ 1.0 decrease) and in BASFI (≥ 0.6 decrease).2 Data were analyzed using nonresponder imputation (NRI), NRI with multiple imputation (NRI-MI) for COVID-19 missing data, and as observed (AO). Sankey diagrams were used to illustrate ASDAS LDA achievement through week 104 using AO and NRI (with AO-NRI as appropriate) analyses.
Results: A total of 460 patients were included in this analysis (bDMARD-naïve r-axSpA, N = 93; bDMARD-IR r-axSpA, N = 211; nr-axSpA, N = 156). Among patients who achieved ASAS40 at weeks 14 and 52, most sustained this response at week 104 (bDMARD-naïve r-axSpA: NRI, 92.3%; AO, 97.3%; bDMARD-IR r-axSpA: NRI-MI, 84.7%; AO, 92.3%; nr-axSpA: NRI-MI, 85.5%; AO, 89.8%) (Fig 1). Most UPA-treated patients who achieved ASDAS LDA at weeks 14 and 52 sustained that response at week 104 (bDMARD-naïve r-axSpA: NRI, 90.0%; AO, 97.3%; bDMARD-IR r-axSpA: NRI-MI, 81.1%; AO, 90.9%; nr-axSpA: NRI-MI, 79.3%; AO, 86.8%). Similar maintenance of response was observed for achievement of MCID in total back pain (NRI: range, 86.7% to 91.9%; AO: range, 92.9% to 100.0%), nocturnal back pain (NRI-MI: range, 89.4% to 92.2%; AO: range, 97.9% to 98.4%), and BASFI (NRI-MI: range, 86.0% to 93.3%; AO: range, 97.0% to 100.0%). In a longitudinal analysis, the long-term sustainability of ASDAS LDA was further examined over time (Fig 2 & 3), demonstrating an increased proportion of patients achieving ASDAS LDA from baseline to weeks 14, 52, and 104.
Conclusion: Patients treated with UPA who showed response at weeks 14 and 52 continued to maintain those responses in disease activity (ASAS40 and ASDAS LDA), total back pain, nocturnal back pain, and physical function at week 104. These findings highlight the consistent and long-term efficacy of UPA across the full spectrum of axSpA.1. Navarro-Compán V, et al. Arthritis Rheumatol. 2023;75 [Abstract].2. Kviatkovsky MJ, et al. J Rheumatol. 2016;43:1680-86.
To cite this abstract in AMA style:
Navarro-Compan V, Mease P, Gensler L, Rudwaleit M, Klionsky Y, Stigler J, Mancl E, Chen S, Urbanik J, Baraliakos X. Sustainability of Clinical Response Through 2 Years Among Upadacitinib-Treated Patients With Axial Spondyloarthritis: Data From the SELECT-AXIS 1 and SELECT-AXIS 2 Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sustainability-of-clinical-response-through-2-years-among-upadacitinib-treated-patients-with-axial-spondyloarthritis-data-from-the-select-axis-1-and-select-axis-2-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustainability-of-clinical-response-through-2-years-among-upadacitinib-treated-patients-with-axial-spondyloarthritis-data-from-the-select-axis-1-and-select-axis-2-trials/

.jpg)
.jpg)