Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Secukinumab, an IL-17A inhibitor, has well-established effectiveness in the treatment of psoriasis (PsO) and psoriatic arthritis (PsA) in both long-term clinical trials and the real world.1,2 There is an intense discussion if biologics can prevent the development of PsA in patients with psoriasis without PsA with nail involvement (higher risk patient group). SERENA is a large, longitudinal, observational study conducted at 438 sites across Europe in adult patients with moderate to severe chronic plaque PsO, active PsA and radiographic axial spondyloarthritis.2 The observations will be put into perspective to historical evidence regarding patients with PsO without PsA not previously treated with biologics3. Here we evaluate the 5-year effectiveness of secukinumab in prevention of PsA.
Methods: Patients received secukinumab treatment for ≥16 weeks before enrolment into the study. In the subgroup of patients with PsO without PsA and nail involvement at the study entry, we analysed the incidence of PsA from inclusion into the study to the 5-year follow up. We describe the outcomes as exposure adjusted incidence rates (EAIR) of developing PsA during the 5-year follow-up, in terms of per 100 patient-years. Descriptive statistics of effectiveness assessments were based on observed data.
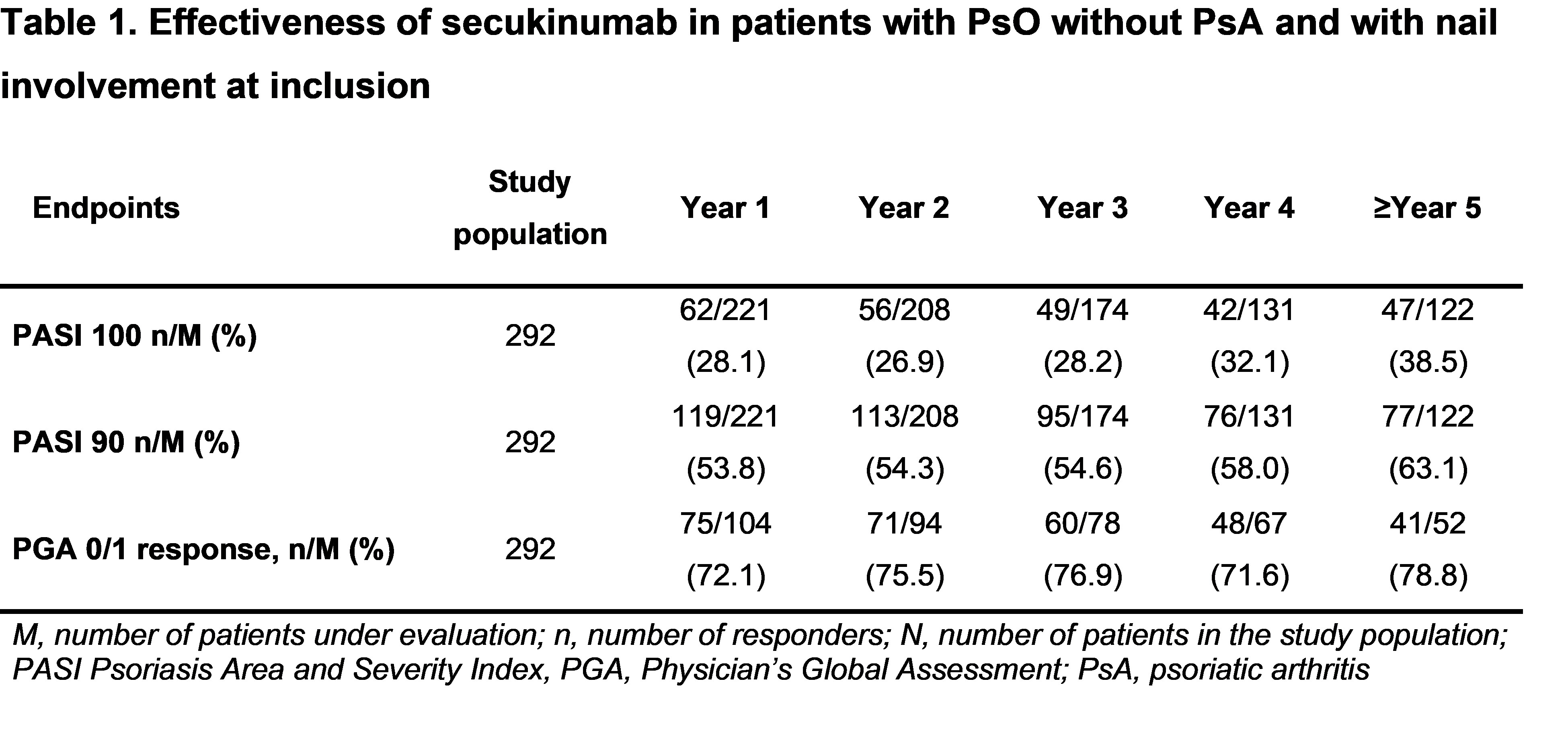
Results: Overall, 292 patients with active PsO with nail involvement without PsA were included in the analysis (mean age at study enrolment, 49.2 years; male, 79.8%; mean body mass index, 29.3 kg/m2; mean time since diagnosis, 17.8 years). 28.8% had previous biologic exposure before the start of secukinumab. After 5 years of study enrolment and a total exposure of 1022 patient-years, most patients (287/292 patients; 98.3%) did not develop PsA. This translates into a yearly, exposure adjusted incidence rate (EAIR) of 0.49 (95% CI: 0.16,1.14). Secukinumab treatment resulted in a sustained control over skin changes as assessed by Psoriasis Area and Severity Index (PASI) and Physician’s Global Assessment (PGA) 0/1 response, with 39% of patients achieving skin clearance (PASI 100) after 5 years (Table 1). Approximately 63% of patients achieved PASI 90 after 5 years (Table 1).
Conclusion: Previously, an 8-year prospective observational study in patients with PsO (including 35% of patients with nail involvement) without PsA reported a yearly incidence rate of PsA of 2.7 per 100 patients not treated with a biologic3. SERENA is one of the largest real-world studies in Europe observing the effect of secukinumab over 5 years. Long-term secukinumab treatment in patients with PsO with nail involvement without PsA resulted in an ~82% reduction of the annual incidence rate of PsA in comparison to historic non-biologic treated patients. These results suggest that secukinumab may be effective in preventing PsA in patients with PsO. Secukinumab-treated patients also achieved sustained long-term control over skin changes.References:1. Blair HA. Drugs 2021;81(4): 483-494. doi: 10.1007/s40265-021-01476-3.2. Kiltz U, et al. Rheumatol Ther. 2022;9(4): 1129-1142. doi: 10.1007/s40744-022-00460-x.3. Eder L. Arthritis Rheumatol. 2016;68(4):915-23. doi: 10.1002/art.39494.
To cite this abstract in AMA style:
Kiltz U, Sfikakis P, Bounas A, Gullick N, LESPESSAILLES E, Brandt-Juergens J, Rashkov R, Vizcaya C, Clemens A, Bao W, Gaffney K. Effective Prevention of Psoriatic Arthritis with Secukinumab: A 5-Year Observation from the SERENA Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effective-prevention-of-psoriatic-arthritis-with-secukinumab-a-5-year-observation-from-the-serena-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effective-prevention-of-psoriatic-arthritis-with-secukinumab-a-5-year-observation-from-the-serena-study/