Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sonelokimab (SLK), a novel Nanobody that binds to both IL-17A and IL-17F with similarly high affinity, is designed to target difficult-to-reach sites of inflammation due to its small size and albumin-binding domain. ARGO was a Phase 2 trial of SLK in active PsA, which met its primary endpoint of ACR50 vs. placebo at Week (W) 12 for SLK 60mg and 120mg (with induction [WI]). Here, we report W24 data in the ARGO trial from a subset of patients naive to bDMARDs (prior biologic use was a stratification factor). We also describe the design of the Phase 3 IZAR-1 trial, which will further evaluate SLK 60mg WI and SLK 60mg with no induction (NI) in this patient population.
Methods: ARGO (NCT05640245) was a 24-week, global, randomized, placebo-controlled, double-blind trial of adults with active PsA (TJC68 ≥3 and SJC66 ≥3). Three SLK doses were assessed: 60mg NI (Q4W), SLK 60mg WI, and SLK 120mg WI (induction doses at W0, 2, 4, and 6; Q4W from W8). In this exploratory analysis, we assessed outcomes at W24 in patients without prior bDMARD exposure treated with SLK 60mg with or without induction (IZAR-1 doses).
Results: Of the 207 patients enrolled in ARGO (Table), 171 patients (82.6%) were naive to bDMARDs. SLK 60mg resulted in clinical responses at W24 in patients naive to bDMARDs (SLK 60mg WI, n=34; SLK 60mg NI, n=33), including ACR50 (SLK 60mg WI: 61.8%; SLK 60mg NI: 63.6%), the higher threshold of ACR70 (SLK 60mg WI: 41.2%; SLK 60mg NI: 45.5%), and the composite endpoint of minimal disease activity ([MDA]; SLK 60mg WI: 58.8%; SLK 60mg NI: 48.5%). For patients naive to bDMARDs with skin involvement (SLK 60mg WI, n=22; SLK 60mg NI, n=28), treatment with SLK resulted in notable PASI 90 (SLK 60mg WI: 81.8%; SLK 60mg NI: 67.9%) and PASI 100 responses (SLK 60mg WI: 68.2%; SLK 60mg NI: 57.1%). SLK was well tolerated, with a safety profile consistent with IL-17 inhibition. Based on these findings, we have designed a 52-week, global, randomized, double-blind, PBO‑controlled, Phase 3 trial in this patient population (IZAR-1; NCT06641076; Figure). An estimated 960 patients will be randomized 1:1:1 to receive SLK 60mg Q4W WI, SLK 60mg Q4W NI, or PBO (SLK 60mg WI from W16). Given the results from ARGO, this sample size should provide sufficient power to demonstrate a statistically significant difference for each SLK arm vs. PBO on the primary outcome of ACR50 at W16. Eligible participants will have a confirmed diagnosis of PsA (CASPAR criteria), active disease (TJC68 ≥3; SJC66 ≥3), currently active or dermatologist-confirmed historical psoriasis, inadequate response to ≥1 conventional DMARD, and ≥1 erosion on hands-and-feet X-ray or hs-CRP > the ULN. Key secondary outcomes (all vs. PBO at W16) include ACR20, PASI 90, and MDA; the PROs HAQ-DI and SF-36 Physical Component Summary; and radiographic progression (vdH-mTSS).
Conclusion: The Phase 2 ARGO study demonstrated high levels of clinical response with SLK, including in patients with active PsA naive to bDMARDs. The randomized Phase 3 IZAR-1 study will further assess outcomes with SLK in patients naive to bDMARDs and is actively recruiting, including at sites across the USA.
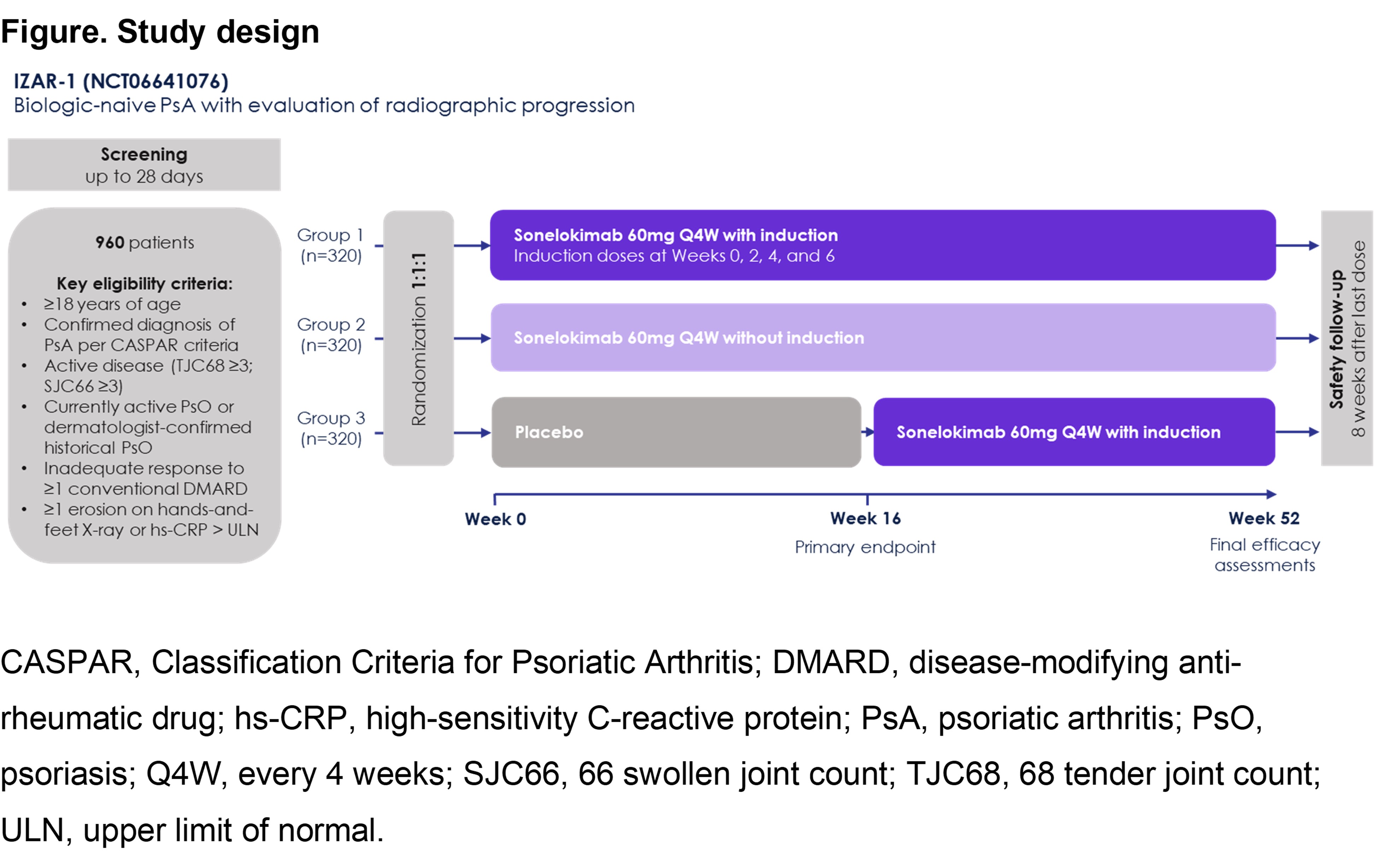 Table. Baseline characteristics from the ARGO trial
Table. Baseline characteristics from the ARGO trial
To cite this abstract in AMA style:
Mease P, Gossec L, Deodhar A, Baraliakos X, Behrens F, Merola J, Eder L, Orbai A, Ramming A, McInnes I, Ogdie A, McGonagle D, Ritchlin C, Brennan N, Porter-Brown B, Cullen E, Thomas M, Albulescu M, Godwood A, Reich K, Coates L. Sonelokimab in Patients With Active Psoriatic Arthritis Who Are Naive to Biologic DMARDs: Phase 2 ARGO Analysis and Phase 3 IZAR-1 Study Design [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sonelokimab-in-patients-with-active-psoriatic-arthritis-who-are-naive-to-biologic-dmards-phase-2-argo-analysis-and-phase-3-izar-1-study-design/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sonelokimab-in-patients-with-active-psoriatic-arthritis-who-are-naive-to-biologic-dmards-phase-2-argo-analysis-and-phase-3-izar-1-study-design/

.jpg)