Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic disease, including psoriasis (Pso) and psoriatic arthritis (PsA), seems to affect male and female patients differently in clinical presentation, disease progression and advanced treatment response1. Sex influence on biologics has been described in bio-naive PsA population2 previously, and this study investigates the sex impact on guselkumab (GUS) persistence in overall, bio-naive and bio-experienced Pso and/or PsA patients.
Methods: This study used data from the French administrative healthcare claims database (SNDS) on all adults with psoriatic disease who received their first GUS dispensation (index date) between 2019 and 2022. Patients were followed until death, treatment discontinuation or the end of the study period (12/31/2023). Two sub-groups were identified in the overall population of patients with a psoriatic disease, using surrogate markers of diagnosis: i) Pso patients (without PsA) and ii) PsA patients (with or without Pso). Treatment persistence for GUS depending on sex (i.e., the duration of treatment from initiation to discontinuation, defined as a drug-free interval of at least 120 days after the last dispensation of GUS) was estimated using the Kaplan-Meier method.
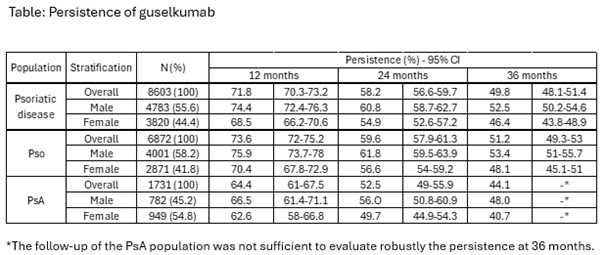
Results: Among the 9,478 patients who received at least one dispensation of GUS between 2019 and 2022, a total of 8,603 adult patients initiated treatment for psoriatic disease: 6,872 (79.9%) were Pso patients and 1,731 (20.1%) PsA patients. Males represented 55.6%, 58.2% and 54.8% of the overall, Pso and PsA populations, respectively. During the 5-year period before GUS initiation, 59.7% of patients had received at least one conventional disease-modifying antirheumatic drug (DMARD) (Pso: 58.2%; PsA: 65.8%), and 61.8% had received at least one biologic DMARD (Pso: 57.2%; PsA: 79.8%). For overall population, persistence rates of GUS treatment at 12 months, 24 months, and 36 months were 71.8%, 58.2%, and 49.8% respectively, with 74.4%, 60.8%, and 52.5% for males and 68.5%, 54.9%, and 46.4% for females. For the Pso population, persistence rates were 73.6% at 12 months, 59.6% at 24 months and 51.2% at 36 months, with respectively 75.9%, 61.8% and 53.4% for males and 70.4%, 56.6% and 48.1% for females. For the PsA group, persistence rates were 64.4% at 12 months and 52.5% at 24 months, with respectively 66.5% and 56.0% for males and 62.6% and 49.7% for females. (Figure & Table). The median times to discontinuation were 35.6 months [95% CI: 33.6-38.0] for the overall population, 40.7 months [95% CI: 37.9-45,8] for males and 30.2 months [95% CI: 28.0-33.0] for females.
Conclusion: Consistent with previous findings showing that TNFi and IL-17i treatment persistence was lower for female PsA patients than for male, our results highlight the sex influence on GUS persistence in psoriatic disease, including PsO and PsA patients. These findings suggest that sex-related factors may influence treatment outcomes, emphasizing the need for tailored treatment strategies to enhance persistence in psoriatic disease management.1Tarannum S et al., Nat Rev Rheumatol. 2022 Sep;18(9):513-5262 Pina Vegas L et al., RMD Open. 2023 Dec 19;9(4):e003570
To cite this abstract in AMA style:
Gossec L, Claudepierre P, Constantin A, Jullien D, Chaalal S, Baraut J, Cipiere L, Gautier L, Lemire P, Passeron T. Influence of Sex on the Therapeutic Persistence of Guselkumab in Psoriatic Disease: a Retrospective National Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/influence-of-sex-on-the-therapeutic-persistence-of-guselkumab-in-psoriatic-disease-a-retrospective-national-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/influence-of-sex-on-the-therapeutic-persistence-of-guselkumab-in-psoriatic-disease-a-retrospective-national-cohort-study/

.jpg)