Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: As a chronic inflammatory disease, PsA is marked by cartilage and bone turnover and articular inflammation. Accordingly, elevated levels of inflammation and bone metabolism are observed in PsA. Zasocitinib (TAK-279) is an oral, allosteric, highly selective and potent tyrosine kinase 2 (TYK2) inhibitor under clinical investigation for PsA treatment. In a phase 2b study in patients with active PsA (NCT05153148), ACR20 response rates were significantly greater with zasocitinib 15 mg or 30 mg once daily versus placebo (PBO) at Week 12 (primary endpoint; both p = 0.002). This study evaluated the longitudinal impact of zasocitinib on biomarkers of cartilage and bone turnover and inflammation in patients with active PsA, including associations between biomarker changes and clinical response.
Methods: In this multicenter, double-blind, PBO-controlled, dose-ranging phase 2b study, patients with active PsA were randomized 1:1:1:1 to zasocitinib 5, 15 or 30 mg or PBO, once daily for 12 weeks. Blood samples were collected at baseline (day 1), Week 4 and Week 12 (pre-dose, if applicable). Plasma biomarkers, including IL-6, colony stimulating factor 1 (CSF1), vascular endothelial growth factor A (VEGF-A), monocyte chemoattractant protein-2 (CCL8), tumor necrosis factor-like weak inducer of apoptosis (TWEAK), type VI collagen degradation marker (C6M), and matrix metalloprotease 3 (MMP3) were measured using the Olink Explore HT or Nordic (C6M) platforms. Longitudinal biomarker changes were modeled using a mixed-effect model, with baseline value, treatment, time and treatment–time interaction as fixed effects and patient as a random effect. Associations with clinical response (ACR20) were measured using absolute area under the receiving operator characteristic curve (abs AUC), adjusted for permutation.
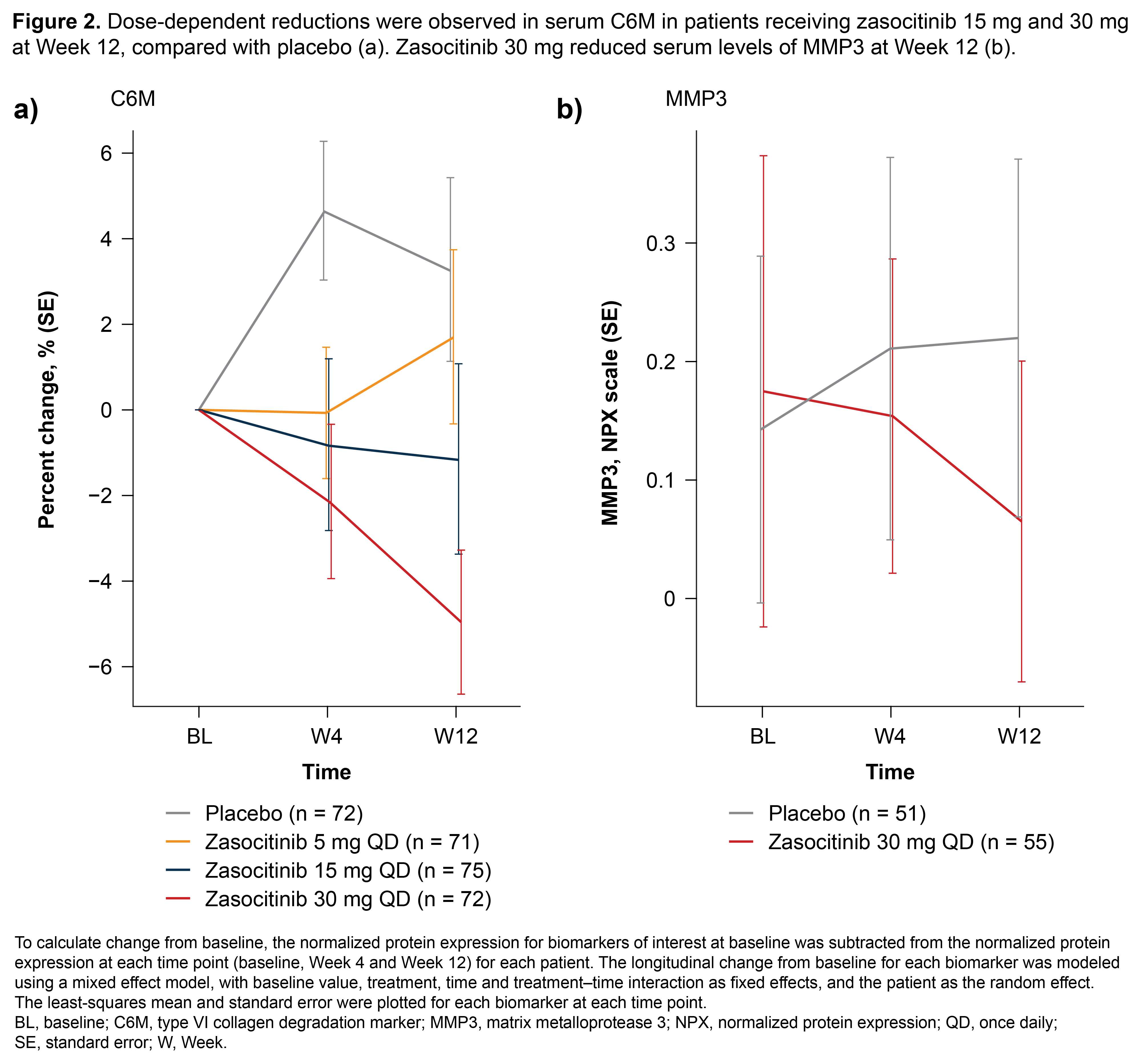
Results: Overall, among 290 treated patients (zasocitinib or PBO), least-squares mean reductions in IL-6 and CSF1 at Week 4 and VEGF-A at Week 12 were associated with ACR20 response (abs AUC: 0.637, 0.627 and 0.637, respectively; p = 0.018, 0.036 and 0.018, respectively). Among ACR20 responders, zasocitinib-treated patients generally showed dose-dependent reductions from baseline in IL-6, VEGF-A, CCL8 and TWEAK at both Weeks 4 and 12 (Figure 1); in contrast, reductions from baseline in these parameters were not consistently observed in ACR20 non-responders, suggesting a link to intercurrent disease activity. At Week 4, dose-dependent C6M reductions were seen in patients receiving zasocitinib 15 mg and 30 mg, with significant reductions versus PBO (0.94-fold [p < 0.05] and 0.93-fold [p < 0.01], respectively); at Week 12 zasocitinib 30 mg had significant reductions in plasma C6M versus PBO (0.92-fold, p < 0.01; Figure 2a) and from baseline (5% reduction, p < 0.01). Zasocitinib 30 mg also reduced plasma MMP3 levels at Week 12 (Figure 2b).
Conclusion: Zasocitinib modulates biomarkers of cartilage and bone turnover and articular tissue inflammation in patients with active PsA as early as Week 4, with changes linked to ACR20 clinical response. Zasocitinib 30 mg significantly reduced bone turnover biomarkers at Week 12 versus PBO. Ongoing phase 3 studies (NCT06671483 and NCT06671496) are examining TYK2 inhibition in PsA.
To cite this abstract in AMA style:
Choudhury A, Cheng J, Hong F, Kumar S, Sugiura A, Tang J, Saha B, Arunachalam V, Hong T, Muensterman E, Wennbo H, Thakker P, McInnes I. Modulation of Soluble Biomarkers of Cartilage and Bone Turnover and Inflammation by Zasocitinib (TAK-279), an Oral, Allosteric, Highly Selective and Potent TYK2 Inhibitor, is Associated with Clinical Response in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/modulation-of-soluble-biomarkers-of-cartilage-and-bone-turnover-and-inflammation-by-zasocitinib-tak-279-an-oral-allosteric-highly-selective-and-potent-tyk2-inhibitor-is-associated-with-clinical/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/modulation-of-soluble-biomarkers-of-cartilage-and-bone-turnover-and-inflammation-by-zasocitinib-tak-279-an-oral-allosteric-highly-selective-and-potent-tyk2-inhibitor-is-associated-with-clinical/

.jpg)