Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Axial spondyloarthritis (axSpA) is a chronic inflammatory disease mainly affecting the sacroiliac joints and spine.1 Peripheral manifestations are common, contributing to disease burden.2 Two clinical axSpA endotypes have been identified by cluster analyses: patients (pts) with predominantly axial disease (i.e., Endotype A), and pts with axial and peripheral manifestations (i.e., Endotype B; associated with higher disease activity and reduced quality of life).3–5 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)‑17F in addition to IL-17A, has shown sustained efficacy and safety to Week (Wk) 104 across the full disease spectrum of axSpA in the phase 3 studies BE MOBILE 1 and 2 and their open-label extension (OLE).6 Here, we report efficacy and safety of BKZ to Wk 104 by endotype to evaluate differences in treatment response.
Methods: Study designs of BE MOBILE 1 (NCT03928704; non-radiographic axSpA), BE MOBILE 2 (NCT03928743; radiographic axSpA) and their OLE (NCT04436640) have been reported previously.7 From Wk 16, all pts received subcutaneous BKZ 160 mg every 4 weeks. At Wk 52, eligible pts could enroll in the OLE.Pts pooled across studies were categorized into endotypes by a validated classification and regression tree (CART) clustering algorithm using baseline characteristics.3Retention and efficacy data to Wk 104 are reported for all randomized pts (Nf586). ASAS40 response and ASDAS major improvement (ASDAS-MI) are reported with non-responder imputation (NRI); ASDAS low disease activity (LDA) and ASDAS change from baseline are reported with multiple imputation (MI). Safety data are reported in pts receiving ≥1 BKZ dose (Nf574).
Results: Of the 586 pts in BE MOBILE 1 and 2 and their OLE, 402 (68.6%) were categorized into Endotype A, and 184 (31.4%) into Endotype B. Baseline characteristics by endotype are shown in the Table. Kaplan-Meier retention rate at Wk 104 was 83.9% in both endotypes. At Wk 16, higher ASAS40 response rates were observed with BKZ vs PBO in both endotypes, sustained to Wk 104 with continuous BKZ (Endotype A: 49.1%; Endotype B: 56.5%; Figure 1A). Trends were largely similar for ASDAS-MI and ASDAS LDA (Figure 1B–C). Reductions from baseline in ASDAS were greater in both endotypes with BKZ vs PBO at Wk 16 and sustained to Wk 104 with continuous BKZ (A: −1.7; B: −2.0; Figure 1D). Greater differences in responses with BKZ vs PBO were observed in Endotype B vs Endotype A. The safety profile of BKZ was consistent across endotypes (treatment-emergent adverse events [TEAEs]: A: 89.1% [352/395], B: 90.5% [162/179]; serious TEAEs: A: 12.2% [48/395], B: 13.4% [24/179]).
Conclusion: BKZ showed comparable retention, efficacy, and safety to Wk 104 in pts with predominantly axial disease and with axial and peripheral manifestations. These data suggest pts with axSpA have improved outcomes with BKZ regardless of endotype.References: 1. Navarro-Compán V. Lancet 2025;405:159–72; 2. De Winter JJ. Arthritis Res Ther 2016;18:196; 3. Costantino F. Rheumatology 2022;61:3289–98; 4. De Craemer A-S. Rheumatology 2022;61:3279–88; 5. Costantino F. Arthritis Rheumatol 2016;68:1660–8; 6. Baraliakos X. Rheumatology 2025;keaf009; 7. Baraliakos X. Ann Rheum Dis 2024;83:199–213.
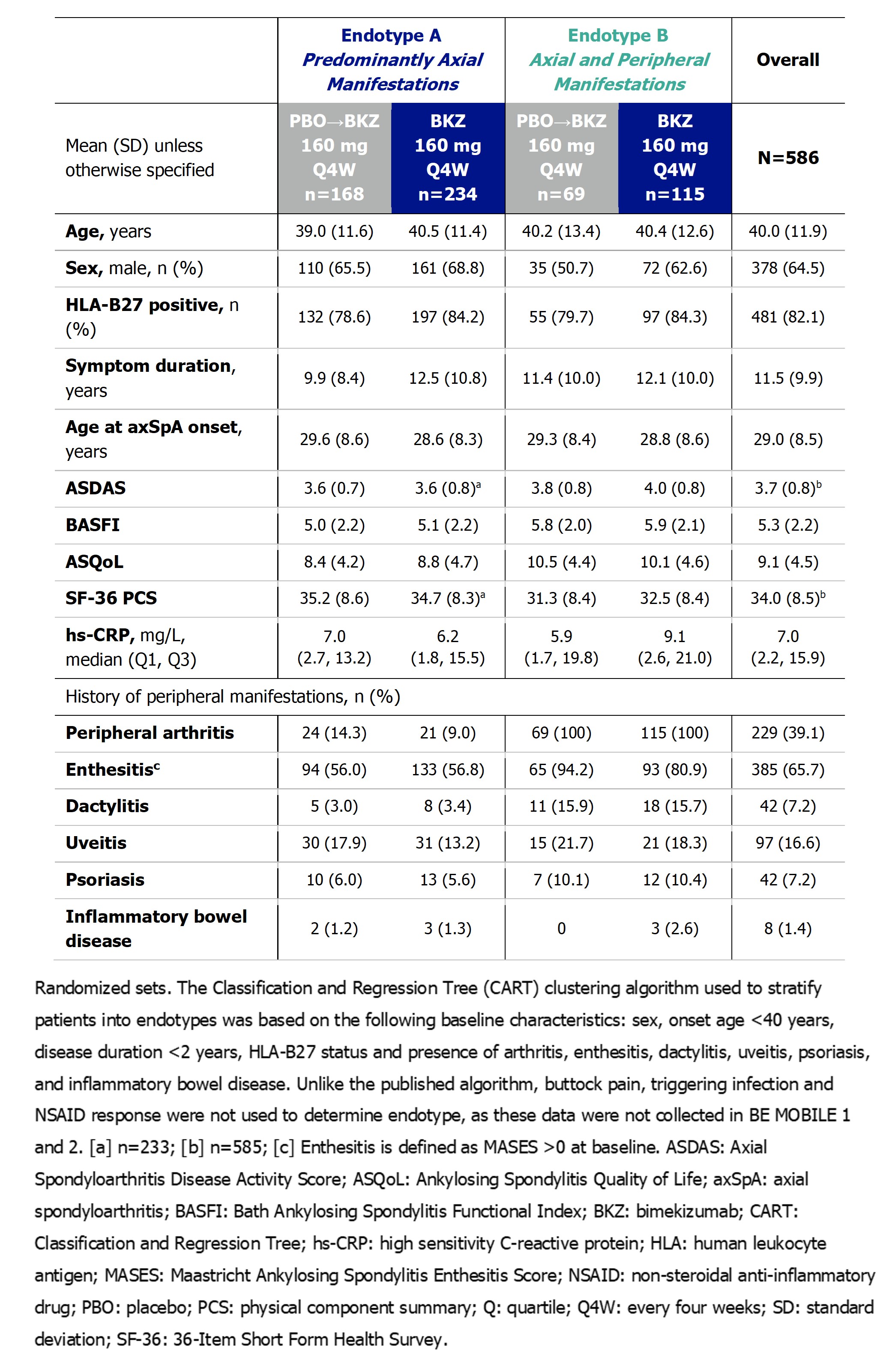 Table. Patient demographics and baseline characteristics, stratified by endotype
Table. Patient demographics and baseline characteristics, stratified by endotype
.jpg) Figure 1. ASAS40, ASDAS-MI, ASDAS LDA and change from baseline in ASDAS to Week 104, stratified by endotype
Figure 1. ASAS40, ASDAS-MI, ASDAS LDA and change from baseline in ASDAS to Week 104, stratified by endotype
To cite this abstract in AMA style:
Costantino F, De Craemer A, Van den Bosch F, Breban M, Taieb V, Voiniciuc D, de Peyrecave N, Elewaut D, D'Agostino M. Similar Efficacy of Bimekizumab in Two Clinical Endotypes of Axial Spondyloarthritis: 2-Year Results from Two Phase 3 Studies and Their Open-Label Extension [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/similar-efficacy-of-bimekizumab-in-two-clinical-endotypes-of-axial-spondyloarthritis-2-year-results-from-two-phase-3-studies-and-their-open-label-extension/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/similar-efficacy-of-bimekizumab-in-two-clinical-endotypes-of-axial-spondyloarthritis-2-year-results-from-two-phase-3-studies-and-their-open-label-extension/
