Session Information
Date: Monday, October 27, 2025
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In patients with axial spondyloarthritis (axSpA), high disease activity is a key indication for biological disease-modifying antirheumatic drug (bDMARD) initiation. The Axial Spondyloarthritis Disease Activity Score (ASDAS) criterion (ASDAS≥2.1) is recommended for defining high disease activity, but the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) criterion (BASDAI≥4) can be used if ASDAS is unavailable [1]. We aimed to compare a newly proposed BASDAI cut-off for high disease activity (BASDAI≥2.5) [2] with ASDAS≥2.1 as an eligibility criterion for bDMARD initiation.
Methods: Prospectively collected real-world data from registries participating in the European Spondyloarthritis (EuroSpA) collaboration were analysed. Patients with axSpA initiating a tumour necrosis factor inhibitor or an interleukin-17A inhibitor as their first bDMARD between 2015 and 2023 were included. Analyses were limited to patients who had complete data on ASDAS and BASDAI at baseline and had attended a 6-month visit. Patients were categorised into 4 subgroups according to baseline ASDAS (≥2.1) and BASDAI (≥2.5) cut-offs for high disease activity. Treatment effect was assessed at 6 months by the Assessment of SpondyloArthritis international Society (ASAS) response measures (ASAS20 and ASAS40). Logistic regression analyses were performed to compare the achievement of treatment effect according to the ASDAS (≥2.1) and BASDAI (≥2.5) cut-offs for high disease activity.
Results: We analysed data from 6,329 patients with axSpA from 9 European registries. Most patients (n=5,753; 91%) fulfilled both ASDAS (≥2.1) and BASDAI (≥2.5) eligibility criteria at baseline, while 243 (4%) patients fulfilled neither of them (Table 1). The number of patients fulfilling either ASDAS or BASDAI criterion alone were 161 (3%) and 172 (3%), respectively. Baseline patient characteristics in the overall cohort and in subgroups are presented in Table 1. The subgroup fulfilling both eligibility criteria demonstrated the highest ASAS20 and ASAS40 responses at 6 months (68% and 52%, respectively) (Table 2). Among patients who only fulfilled the ASDAS or BASDAI criterion alone, similar ASAS20 (37% and 39%) and ASAS40 (17% and 15%) responses were achieved. The results of the regression analyses showed that fulfilling either ASDAS or BASDAI criterion was significantly associated with achieving ASAS20 and ASAS40 responses (Figure 1). When both criteria were included in the regression analyses, ASDAS≥2.1 and BASDAI≥2.5 were positive predictors of treatment effect at 6 months.
Conclusion: In a multinational European cohort, similar numbers of patients were eligible for treatment with bDMARDs when applying either ASDAS≥2.1 or BASDAI≥2.5 as the eligibility criterion. Patients with ASDAS≥2.1 and BASDAI≥2.5 demonstrated the best response to bDMARD treatment. These findings support the use of BASDAI≥2.5 as an indication for biologic treatment in axSpA, particularly when ASDAS is not available. References1. Ramiro et al. (2023). Ann Rheum Dis, 82(1), 19-34.2. Georgiadis et al. (2025). Under review.
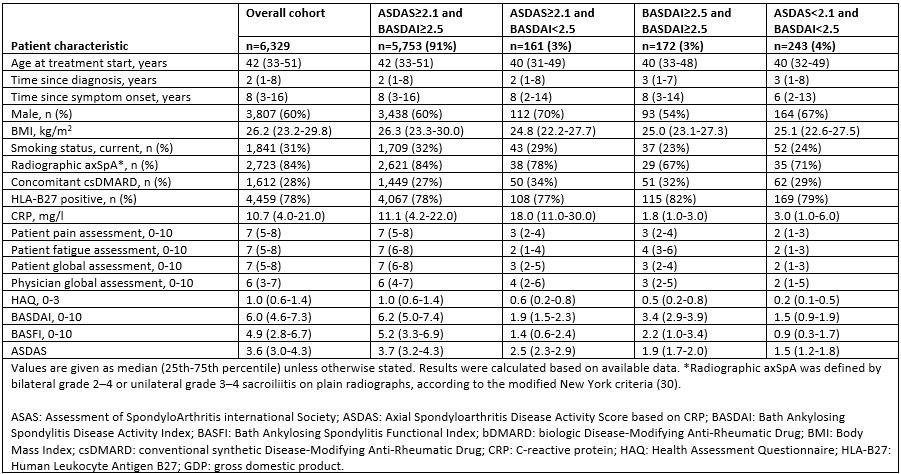 Table 1. Baseline patient characteristics in overall cohort and in subgroups according to baseline ASDAS and BASDAI cut-off values for high disease activity
Table 1. Baseline patient characteristics in overall cohort and in subgroups according to baseline ASDAS and BASDAI cut-off values for high disease activity
.jpg) Table 2. Treatment effect at 6 months in overall cohort and in subgroups according to baseline ASDAS and BASDAI cut-off values for high disease activity
Table 2. Treatment effect at 6 months in overall cohort and in subgroups according to baseline ASDAS and BASDAI cut-off values for high disease activity
.jpg) Figure 1. Forest plots of odds ratios with 95% confidence intervals for achieving ASAS 20 and ASAS40 at 6 months derived by logistic regression models including I. ASDAS criterion alone, II. BASDAI critetion alone, and III. both ASDAS and BASDAI criteria. Logistic regression analyses were adjusted for sex, age and GDP per capita (1,000$), as a proxy for socio-economic status of registries. ASAS: Assessment of SpondyloArthritis international Society; ASDAS: Axial Spondyloarthritis Disease Activity Score based on CRP; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; CI: confidence interval; OR: odds ratio.
Figure 1. Forest plots of odds ratios with 95% confidence intervals for achieving ASAS 20 and ASAS40 at 6 months derived by logistic regression models including I. ASDAS criterion alone, II. BASDAI critetion alone, and III. both ASDAS and BASDAI criteria. Logistic regression analyses were adjusted for sex, age and GDP per capita (1,000$), as a proxy for socio-economic status of registries. ASAS: Assessment of SpondyloArthritis international Society; ASDAS: Axial Spondyloarthritis Disease Activity Score based on CRP; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; CI: confidence interval; OR: odds ratio.
To cite this abstract in AMA style:
Georgiadis S, Ørnbjerg L, Michelsen B, Kvien T, Shoae Kazemi M, Glintborg B, Loft A, Fonseca R, Santos H, Reich A, Regierer A, Rutanen J, Kuusalo L, Macfarlane G, Jones G, Ciurea A, Nissen M, Gudbjornsson B, Palsson O, Rotar Z, Perdan Pirkmajer K, Di Giuseppe D, Hetland M, Ostergaard M. Redefining BASDAI cut-offs: implications for patients’ eligibility for initiating biologic disease-modifying antirheumatic treatment in axial spondyloarthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/redefining-basdai-cut-offs-implications-for-patients-eligibility-for-initiating-biologic-disease-modifying-antirheumatic-treatment-in-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/redefining-basdai-cut-offs-implications-for-patients-eligibility-for-initiating-biologic-disease-modifying-antirheumatic-treatment-in-axial-spondyloarthritis/
