Session Information
Date: Monday, October 27, 2025
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: For women with Sjögren’s disease, the risk of Fetal Atrioventricular Block (fAVB) is a critical aspect in pregnancy counseling. To date, the most reliable predictor of fAVB is a previously affected child, followed by high titer Anti-SSA/Ro52 and 60 antibody titers. In advising on risk for women without an affected child, physicians face the challenge of interpreting different reporting units and upper limits of commercial assay values. Accordingly, this current study leveraged a prospective, anti-SSA/Ro positive pregnancy cohort to compare results obtained from commercial laboratories across the U.S. to those measured in a central research laboratory. The goal was to identify commercial values that would associate with a low risk of fAVB and reduce the need for standard of care surveillance.
Methods: Seven hundred and sixty-six pregnant subjects from 24 centers who tested positive for anti-SSA/Ro (either anti-Ro52 or anti-Ro60 or both) in their local commercial assay were referred to STOP BLOQ for prospective evaluation by home fetal Doppler to rapidly identify emergent fAVB. Eight laboratories were included in this analysis, each testing at least 10 subjects (capturing 526 study participants). The values and descriptors of ranges are provided in Table 1. Each sample was tested for anti-Ro52kD (recombinant antigen) and Ro60kD (native antigen) in the central NYU research lab at serial dilutions ranging from 1:100 to 1:100,000. A threshold titer for risk of fAVB was set at 1,000 ELISA units (EU) for either anti-Ro52kD or Ro60kD based on the evaluation of 50 subjects with a child affected by fAVB (Buyon, A&R, 2023). None of the 10 cases of fAVB in STOP BLOQ were < 1000 EU for either anti Ro52kd or 60kD.
Results: Only one of the commercial laboratories, ARUP (tested in 74), provided a linear range of positivity for both anti Ro52kD and Ro60kD. Previous studies identified a risk threshold at 110 arbitrary units (AU) for each specificity (Kaizer, AJOG, 2022). For anti-Ro52, 29 were > 110 AU and all but one was > 1000 EU. For anti-Ro60 44 were > 110 AU and all but 6 were > 1000 EU. Overall, the values for ARUP and NYU were strongly correlated (Table 2). Seven of the 8 commercial laboratories provided a negative cutoff and an upper threshold for positivity. Three hundred and fifty-four patients were tested using the BioPlex assay. Of 81 below the upper limit > 8, 66 did not meet the research risk threshold and none had an affected child. Of 273 above the upper limit, 224 exceeded the threshold risk of 1000 EU for either anti-Ro52kD or 60kD (PPV: 82%; NPV: 81%), 5 of which developed fAVB. Twenty-one pregnant subjects were tested in the Mayo labs. Of 10 below the commercial lab upper limit, 8 did not meet the research risk threshold. Of the 11 that exceed the commercial lab upper limit, all exceed the research risk threshold. Values for the 6 other commercial labs are detailed in Table 1.
Conclusion: These data provide a framework for evaluating commercial values to guide fetal surveillance of anti-SSA/Ro pregnancies. However, there are inconsistencies in the ability of a test result to associate with risk, with most labs demonstrating that anti-SSA/Ro below the positive upper limit are not at risk, but for a few, predictions are less reliable.
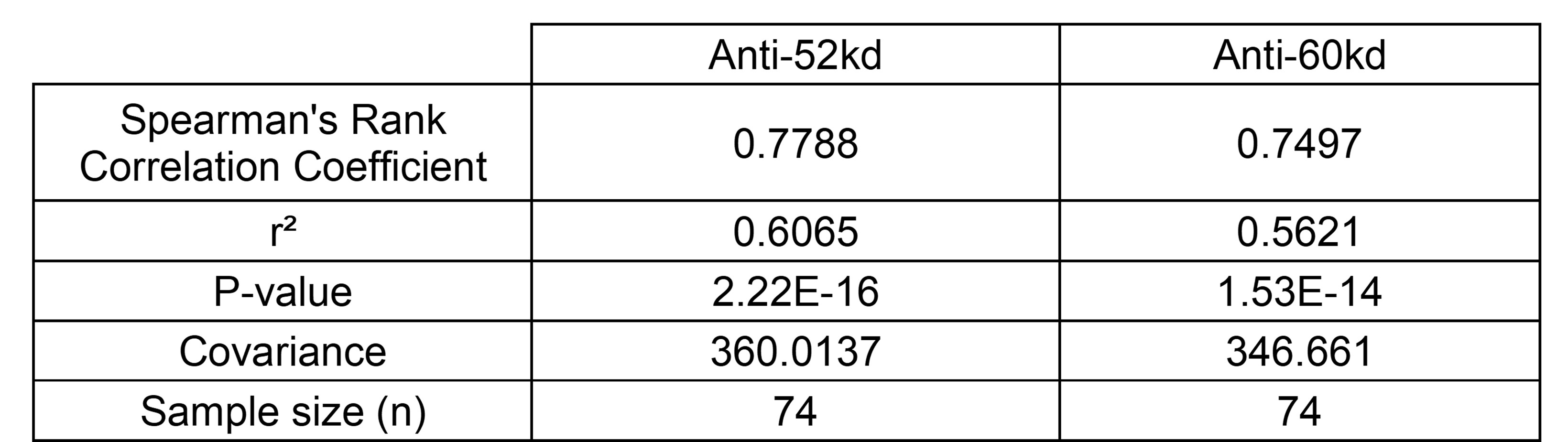 Table 1: Anti-SSA/Ro Testing Across Eight Laboratories.
Table 1: Anti-SSA/Ro Testing Across Eight Laboratories.
.jpg) Table 2: Spearman’s Rank Correlation Coefficient Between ARUP Laboratory and NYU Research Laboratory.
Table 2: Spearman’s Rank Correlation Coefficient Between ARUP Laboratory and NYU Research Laboratory.
To cite this abstract in AMA style:
Masson M, Cuneo B, Carlucci P, Izmirly P, Brandt J, Phoon C, Roman A, Saxena A, Belmont M, Penfield C, Lee Y, Nusbaum J, Rubenstein A, Sinkovskaya E, Abuhamad A, Satou G, Hogan W, Moon-Grady A, Howley L, Donofrio M, Levasseur S, Geiger M, Owens S, Cumbermack K, Matta J, Joffe G, Lindblade C, Haxel C, Kohari K, Copel J, Strainic J, Doan T, Holloman C, Killen S, Tacy T, Kaplinski M, Sachan N, Fraser N, Clancy R, Buyon J. Assessment of Anti-SSA/Ro Testing Across Various Commercial Laboratories in the United States to Reduce the Burden of Surveillance in Pregnant Women with No History of Fetal Atrioventricular Block (fAVB) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessment-of-anti-ssa-ro-testing-across-various-commercial-laboratories-in-the-united-states-to-reduce-the-burden-of-surveillance-in-pregnant-women-with-no-history-of-fetal-atrioventricular-block-fa/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-anti-ssa-ro-testing-across-various-commercial-laboratories-in-the-united-states-to-reduce-the-burden-of-surveillance-in-pregnant-women-with-no-history-of-fetal-atrioventricular-block-fa/
