Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus kinase (JAK) inhibitors are recommended for patients with rheumatoid arthritis (RA) who have an inadequate response to conventional synthetic or biologic disease-modifying antirheumatic drugs (csDMARDs or bDMARDs). However, evidence is limited regarding optimal treatment strategies following JAK inhibitor failure. This study aimed to evaluate real-world treatment patterns after JAK inhibitor discontinuation and to compare the efficacy and drug retention rates among different bDMARD classes in this context.
Methods: This retrospective study included patients with RA who discontinued JAK inhibitors due to inefficacy or intolerance between August 2015 and March 2025 and subsequently switched to an alternative bDMARDs. Changes in disease activity, as measured by the Disease Activity Score in 28 joints using ESR (DAS28-ESR), were assessed at 6 months post-switch, and drug retention rates were compared across three bDMARD groups: TNF inhibitors, IL-6R inhibitors, and other bDMARDs. Low disease activity (LDA) was defined as DAS28-ESR score < 3.2.
Results: Of 151 patients who discontinued JAK inhibitors, 59 (39.1%) switched to TNF inhibitors (adalimumab Nf33, etanercept Nf16, golimumab Nf9, infliximab Nf1), 69 (45.7%) to IL-6R inhibitors, and 23 (15.2%) to other bDMARDs (abatacept Nf19, rituximab Nf4). Among them, 113 patients (40 TNF inhibitor group, 53 IL-6R inhibitor group, and 20 other bDMARD group) remained on therapy for at least 6 months and were included in the efficacy and drug retention analyses. Baseline characteristics were generally comparable across the groups, although patients in other bDMARDs group were older and had a higher number of prior bDMARDs. Baseline DAS28-ESR was also significantly higher in the other bDMARD group than in the IL-6R inhibitor group (p = 0.012). At 6 months, patients who switched to IL-6R inhibitors showed significantly greater improvement in DAS28-ESR compared to those who switched to TNF inhibitors (adjusted p = 0.027). No significant differences were observed between IL-6R inhibitors and other bDMARDs (adjusted p = 0.378), or between TNF inhibitors and other bDMARDs (adjusted p =1.000). LDA was achieved in 83.0% (44/53) of the IL-6R inhibitor group, compared to 62.5% (25/40) in the TNF inhibitor group and 60.0% (12/20) in the other bDMARD group. The overall group difference was statistically significant (p = 0.042), but pairwise comparisons did not remain significant after Bonferroni correction (TNF vs. IL-6R: adjusted p = 0.075; TNF vs. other bDMARDs: adjusted p = 1.000; IL-6R vs. other bDMARDs: adjusted p = 0.114). Drug retention rates did not differ significantly among the three groups (p = 0.249).
Conclusion: In this real-world cohort of RA patients who discontinued JAK inhibitors, IL-6R inhibitors were the most frequently selected subsequent therapy and were associated with greater clinical improvement at 6 months compared to TNF inhibitors. However, drug retention rates were comparable across all bDMARD classes. These findings provide insight into treatment decisions and outcomes following JAK inhibitor failure in RA management.
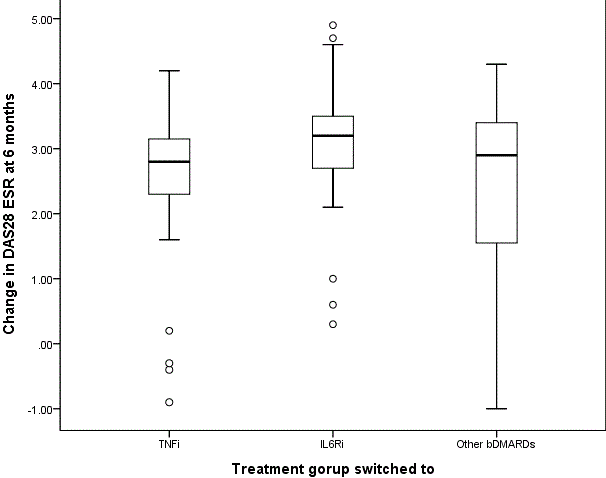 Figure 1. Change in DAS28-ESR over 6months following bDMARD switch after JAK inhibitor discontinuation. Statistical comparisons between groups were performed using the Mann–Whitney U test with Bonferroni correction. Adjusted p-values: 0.027 (TNF inhibitor vs. IL-6R inhibitor), 0.378 (IL-6R inhibitor vs. other bDMARDs), and 1.000 (other bDMARDs vs. TNF inhibitor).
Figure 1. Change in DAS28-ESR over 6months following bDMARD switch after JAK inhibitor discontinuation. Statistical comparisons between groups were performed using the Mann–Whitney U test with Bonferroni correction. Adjusted p-values: 0.027 (TNF inhibitor vs. IL-6R inhibitor), 0.378 (IL-6R inhibitor vs. other bDMARDs), and 1.000 (other bDMARDs vs. TNF inhibitor).
To cite this abstract in AMA style:
Ko S, Kim Y, Ahn S, Oh J, Kim Y, Lee C, Yoo B, Hong S. Treatment Patterns and Outcomes After Janus Kinase Inhibitor Discontinuation in Rheumatoid Arthritis: A Real-World Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/treatment-patterns-and-outcomes-after-janus-kinase-inhibitor-discontinuation-in-rheumatoid-arthritis-a-real-world-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-and-outcomes-after-janus-kinase-inhibitor-discontinuation-in-rheumatoid-arthritis-a-real-world-cohort-study/
