Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Early rheumatoid arthritis (RA) is a critical therapeutic window where prompt treatment targeting remission or low disease activity can significantly reduce long-term disability and systemic complications. Methotrexate (MTX) remains the standard of care, but interest in complementary approaches, particularly Ayurveda—a WHO-recognized traditional Indian medical system with anti-inflammatory and immunomodulatory properties—is increasing. A prior pilot RCT suggested potential benefit of Ayurvedic formulations when combined with MTX, without added toxicity. We conducted a double-blind, randomized controlled trial to evaluate the efficacy and safety of combining Ayurvedic treatment with methotrexate versus methotrexate with placebo in early RA, hypothesizing greater benefit with combination therapy in early disease.
Methods: This exploratory, double-blind RCT was conducted over 15 months (Aug 2023–Dec 2024) in patients fulfilling 2010 ACR/EULAR RA criteria with ≤1 year disease duration. Patients (n=140) were randomized into two groups: MTX + Ayurveda (n=70) or MTX + Placebo (n=70). Ayurvedic treatment included Panchkol churna, Erand oil, Rasna Saptak Kwath, and Singhnad guggul administered sequentially alongside stable MTX dosing (15–25 mg/week). Blinding was maintained for participants and outcome assessors. Data were represented in mean (SD) and analysed as treated. Fisher’s exact test and Chi-Square tests were used for categorical variables. Group comparisons were done at baseline and 20 weeks using t-tests and Wilcoxon-Mann Whitney U Test for parametric and nonparametric data respectively (figure 1).
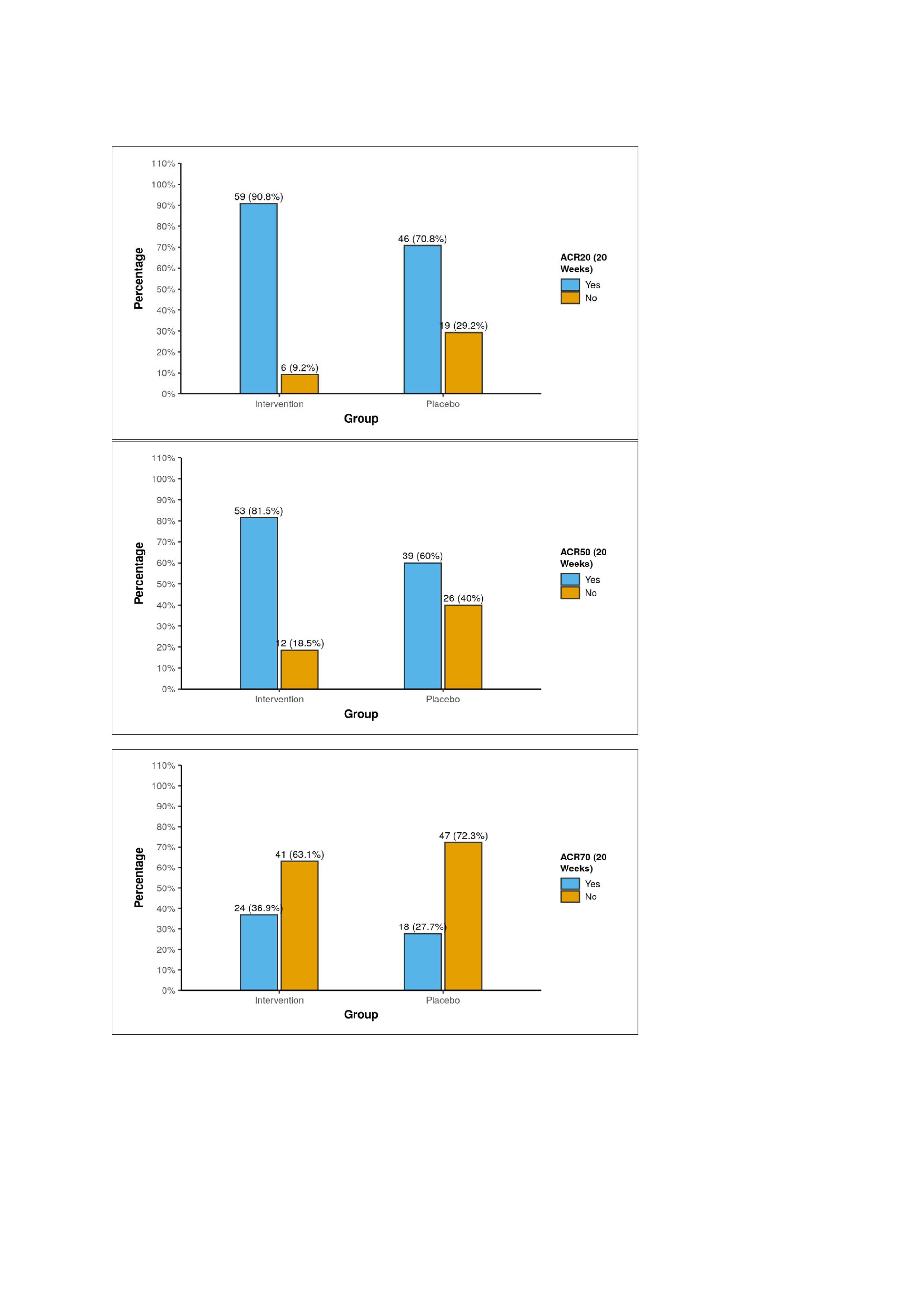
Results: Of 140 enrolled, 130 patients completed 20 weeks. There was no statistical significant difference among two groups in terms of baseline disease activity including age, rheumatoid factor and anti-CCP positivity. At 20 weeks, numerically more patients in the Ayurveda + MTX (49.2%) arm achieved remission as compared to placebo+ MTX (33.8%) by DAS28CRP, though between-group differences were not statistically significant (Figure 2). However, mean reduction in DAS28-CRP, DAS28-ESR, CDAI, and SDAI scores from baseline to 20 weeks was significantly greater in the combination arm (p < 0.05 for all). ACR20 and ACR50 response rates were also significantly higher in the Ayurveda + MTX group (ACR20: 90.8% vs 70.8%, p=0.007; ACR50: 81.5% vs 60.0%, p=0.012) (Figure 3). Adverse events, mostly gastrointestinal, were comparable between groups and mild.
Conclusion: In early RA, adjunctive Ayurvedic therapy with methotrexate led to significantly greater improvements in disease activity and ACR response rates over 20 weeks, with a favorable safety profile. These findings support further investigation of integrative approaches in early disease stages, balancing traditional systems with modern clinical rigor to optimize patient outcomes.
 Comparison of disease activity, remission and response rate at 20 weeks between intervention and placebo groups
Comparison of disease activity, remission and response rate at 20 weeks between intervention and placebo groups
.jpg) Remission rate between two groups as per DAS28CRP composite disease activity index
Remission rate between two groups as per DAS28CRP composite disease activity index
.jpg) ACR response rate between two groups
ACR response rate between two groups
To cite this abstract in AMA style:
Sethi P, Pai V, Ranka R, Gupta A, Baweja A, sangwan r, Rai A, Punjadath S, Maskani N. Efficacy Of Ayurvedic Treatment Versus Placebo, Each In Combination With Methotrexate In Early RA Over 20 Weeks: An Exploratory Randomized Control Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-ayurvedic-treatment-versus-placebo-each-in-combination-with-methotrexate-in-early-ra-over-20-weeks-an-exploratory-randomized-control-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-ayurvedic-treatment-versus-placebo-each-in-combination-with-methotrexate-in-early-ra-over-20-weeks-an-exploratory-randomized-control-trial/
