Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) significantly impacts quality of life in multiple domains. This study aimed to evaluate the impact of initial biologic/targeted synthetic disease-modifying anti-rheumatic drug (b/tsDMARD) therapy on sexual function in women with RA and sought to assess changes in sexual function over six months of treatment and identify clinical and laboratory predictors of these changes.
Methods: Women with RA indicated for b/tsDMARD therapy within the Czech ATTRA registry were recruited. Participants completed the Female Sexual Function Index (FSFI; higher scores indicate better sexual function) at baseline (M0) and after six months (M6). Routine clinical and laboratory data were collected, with patient-reported outcomes regarding function and quality of life (Health Assessment Questionnaire (HAQ), 36-Item Short Form Health Survey (SF-36), EuroQoL 5-Dimension Questionnaire (EuroQoL), and Multidimensional Health Assessment Questionnaire (MDHAQ)). Comparisons were performed using the Wilcoxon test, p-values adjusted for multiple comparisons using the Benjamini-Hochberg method. Selection of baseline predictors with improvement in IIEF domains was performed using LASSO regression. Associations with FSFI improvement were assessed using bivariate screening.
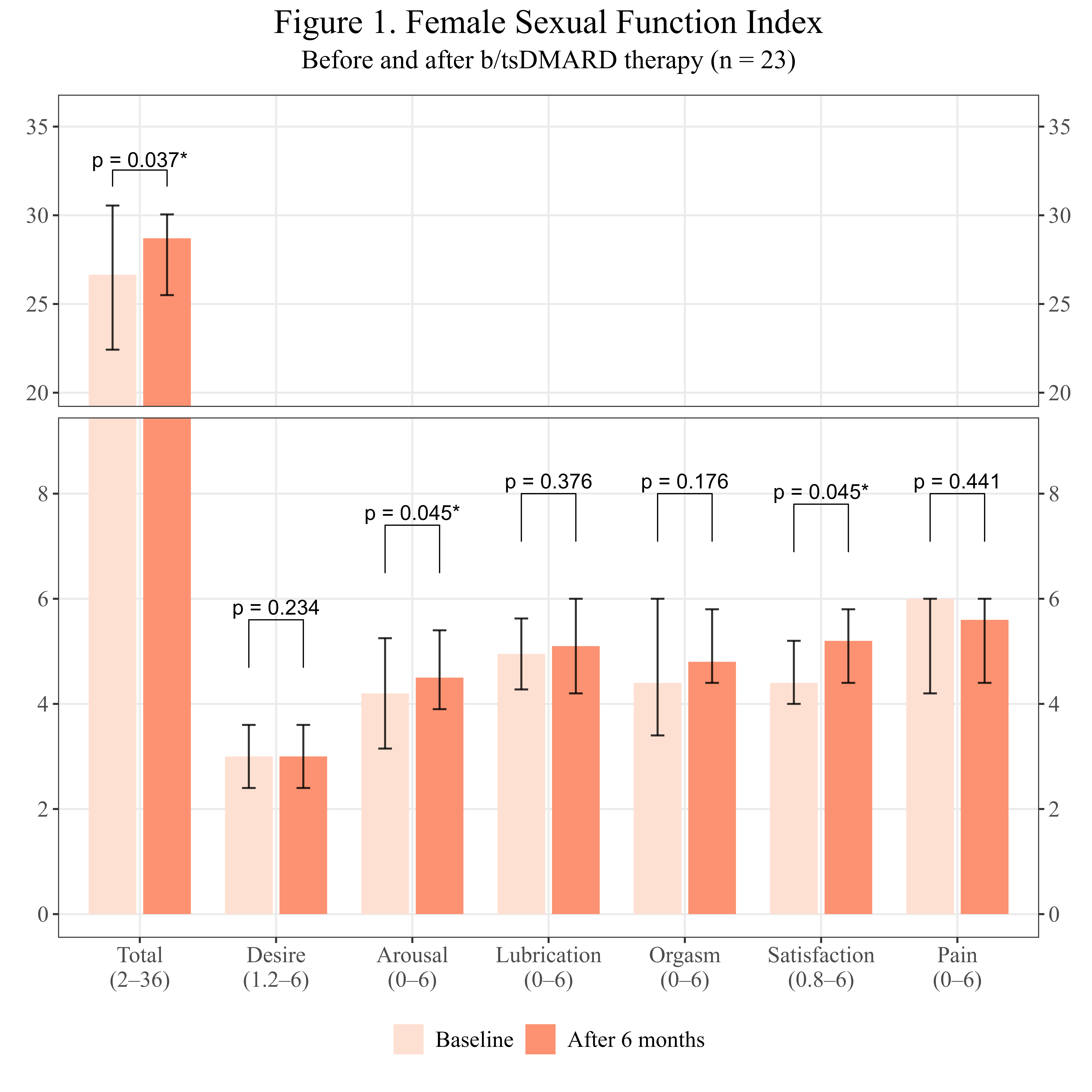
Results: Out of 36 screened patients, 23 sexually active women completed the questionnaires at both time intervals (Table 1). The baseline prevalence of sexual dysfunction was 47% (defined as the published cutoff of FSFI Total < 26.55).At the 6-month follow-up, b/tsDMARD therapy had a significant positive effect on inflammation (ESR, CRP), disease activity (DAS28-ESR, DAS28–CRP), function, and quality of life (HAQ, MDHAQ, SF-36, and EuroQoL) (p < 0.05 for all). Regarding sexual function, we observed small but significant benefits in the FSFI Total Score (from 26.65 to 28.7, p=0.037), as well as in the domain of Arousal (from 4.2 to 4.5, p=0.0453) and Satisfaction (from 4.4 to 5.2, p=0.0453) (Figure 1). The 6M follow-up prevalence of sexual dysfunction was 30%In bivariate analysis, improvements in sexual function correlated with reduced inflammation (CRP), lower disease activity (DAS28-CRP, DAS28-ESR, SDAI), and improved function/quality of life (HAQ, EuroQoL, SF-36).LASSO regression identified baseline FSFI domain scores as the strongest predictors of improvements in sexual function. However, weaker but relevant predictors were also observed (Table 2).
Conclusion: Women with RA experienced modest but significant improvements in sexual function (particularly in the domain of Arousal and Satisfaction) after six months of initial b/tsDMARD therapy. These changes were associated with reduced inflammation, better physical function, and improved quality of life. Further research is needed to confirm these associations and elucidate underlying mechanisms..Supported by MHCR 023728
To cite this abstract in AMA style:
Zavada D, Vala B, Navratilova A, Losterova V, Moravcova L, Vencovsky J, Šenolt L, Pavelka K, Tomcik M. Initial Biologic Therapy Improves Sexual Function in Female Patients with Rheumatoid Arthritis: A 6 Month Follow-Up Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/initial-biologic-therapy-improves-sexual-function-in-female-patients-with-rheumatoid-arthritis-a-6-month-follow-up-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initial-biologic-therapy-improves-sexual-function-in-female-patients-with-rheumatoid-arthritis-a-6-month-follow-up-study/

.jpg)
.jpg)