Session Information
Date: Monday, October 27, 2025
Title: (1306–1346) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Though the efficacy and safety profile of tofacitinib in RA has been established in prior trials, the effectiveness of tofacitinib in routine care settings compared to biologic DMARDs (bDMARDs) is limited.This observational cohort study of RA patients (pts) evaluated the real-world effectiveness of tofacitinib compared to select bDMARDs overall and within relevant subgroups of demographic and clinical characteristics using data from OM1 PremiOM™ RA (OM1, Boston, MA), a multisource dataset with linked claims and EHR data on RA pts in the US.
Methods: Pts with RA aged ≥18 years initiating tofacitinib or select bDMARDs (tumor necrosis factor inhibitors (TNFi); abatacept; interleukin 6 inhibitors (IL-6i)) from 01 January 2013 – 13 March 2024 and with ≥1 Clinical Disease Activity Index (CDAI) score in 45 days prior to and including the index date were included. Stabilized inverse probability treatment weights (sIPTW) were calculated to adjust for demographics, treatment history, and comorbidities). Crude incidence rates (IRs) per 1000 pt-years (PYs) were calculated to estimate the first record of CDAI remission (≤ 2.8) measured at 6- and 12-months. Cox regression with robust standard errors were used to calculate weighted hazard ratios (HRs) and 95% confidence intervals (CIs) overall and stratified by baseline CDAI >/≤ 10. Subgroup analyses by pre-specified variables of interest were conducted to assess heterogeneity of treatment effect (HTE) among pts with baseline CDAI >10.
Results: In total, 21,340 pts were included (tofacitinib, Nf3,681; TNFi, Nf11,667; abatacept, Nf3,528; IL-6i, Nf2,464) (Table). Mean age at index date ranged from 58-62 years, and proportion with disease duration ≥ 2 years ranged from 48.7%-70.0%. At baseline, 36.6% of pts initiating tofacitinib had used ≥ 2 prior biologic or targeted synthetic DMARDs (b/tsDMARDs) vs TNFi (13.5%), abatacept (31.2%) and IL-6i (49.6%). Comorbidities and CDAI scores at baseline were similar between cohorts. Treatment switch rates were higher among tofacitinib (5.7-12.9%) vs. comparator (1.7-3.5%) pts at 6- and 12-months, while treatment discontinuation rates were slightly lower (33.8-51.6% vs. 36.6-63.0%).In the main sIPTW analysis, there were no statistically significant differences in the rate of achieving remission between treatments at 6- and 12-months (Fig 1). Results were consistent when stratifying pts by baseline CDAI >/≤ 10, apart from a lower rate of remission for tofacitinib compared to IL-6i at 12 months among those with baseline CDAI >10 (HR = 0.54, 95% CI 0.35-0.83). Subgroup analyses suggested HTE among pts with obesity (BMI ≥30kg/m2), ≥2 prior biologic or targeted synthetic DMARDs, prior corticosteroid use, and Sjogren’s syndrome for tofacitinib compared to TNFi among pts with baseline CDAI >10 at 6 months (p-interaction < 0.05), but not 12 months (Fig 2).
Conclusion: In this large cohort study using real-world data, no significant differences were found in achieving remission between treatments among the overall RA population. Subgroup analyses suggest some potential HTE; however, interpretation is limited by low event rates, missing data, and potential residual confounding.
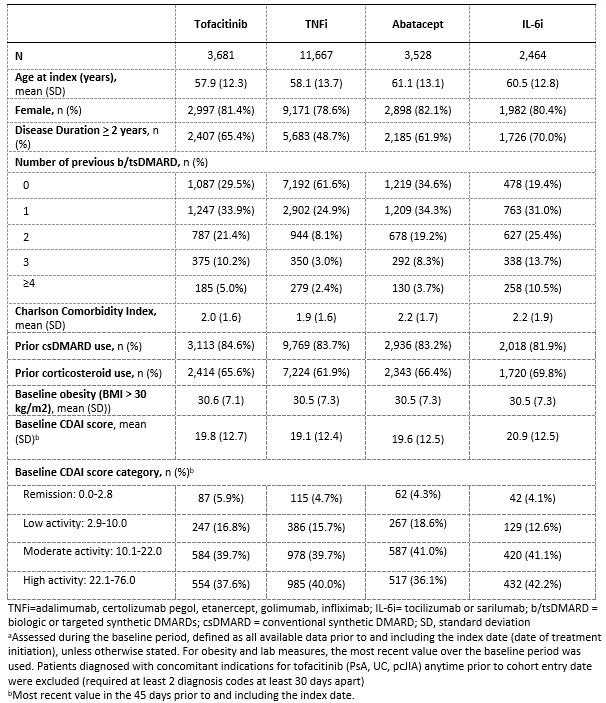 Table. Demographics/clinical characteristics of RA patients initiating treatment, before propensity score weighting
Table. Demographics/clinical characteristics of RA patients initiating treatment, before propensity score weighting
.jpg) Figure 1. Effectiveness of tofacitinib vs bDMARDs in achieving remission (CDAI ≤ 2.8) at 6 and 12 months
Figure 1. Effectiveness of tofacitinib vs bDMARDs in achieving remission (CDAI ≤ 2.8) at 6 and 12 months
.jpg) Figure 2. Effectiveness of tofacitinib vs TNFi in achieving remission (CDAI ≤ 2.8) at 6 and 12 months, by subgroup.
Figure 2. Effectiveness of tofacitinib vs TNFi in achieving remission (CDAI ≤ 2.8) at 6 and 12 months, by subgroup.
To cite this abstract in AMA style:
Liao K, Gopalakrishnan C, Yazdany J, Bell G, Mothi S, Gauthier G, Yndestad A, Gianfrancesco M. Effectiveness of new initiators of tofacitinib and other biologic/targeted synthetic DMARDs in patients with rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-new-initiators-of-tofacitinib-and-other-biologic-targeted-synthetic-dmards-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-new-initiators-of-tofacitinib-and-other-biologic-targeted-synthetic-dmards-in-patients-with-rheumatoid-arthritis/
