Session Information
Date: Monday, October 27, 2025
Title: (1306–1346) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin (IL)-6 plays an important role in the pathogenesis of depression in rheumatoid arthritis (RA) patients. IL-6 inhibitors used to treat patients with RA may also have an antidepressant effect. The objective of this study is to evaluate the effectiveness of 24-week IL-6 inhibitor therapy with olokizumab (OKZ) in combination with or without psychopharmacotherapy (PPT) in patients with moderate to high RA activity.
Methods: According to ICD-10, a psychiatrist diagnosed varying severity of depression (chronic or recurrent) in all patients during a semi-structured interview. At week 0, all patients were randomized using sequential numbers in a 2:2:1 ratio into one of three groups: in the group 1, patients received conventional synthetic disease modifying antirheumatic drugs (csDMARDs)+OKZ 64 mg subcutaneously once every 4 weeks (q4w) (n=49); in the group 2, patients received csDMARDs+OKZ 64 mg subcutaneously q4w along with PPT (n=51); in the group 3, patients received csDMARDs + PPT (n=25). The study duration was 24 weeks. The severity of depression was assessed using the PHQ-9 and MADRS scales; while anxiety was assessed using the HAM–A scale. Projective experimental psychological techniques were also used.
Results: A total of 125 patients with RA were included, 102 (81,6%) of them being women. The average age of the patients was 48,5±12,6 years; the majority of the patients (86.4%) had high RA activity and stable 12-week therapy with csDMARDs was ineffective . Additionally, 34 (27,2%) patients had shown inefficacy of one or more biological DMARDs. After 12 and 24 weeks of therapy, a significant decrease in the severity of depression and anxiety was observed in all patients’ groups. However, the differences between the final and initial values of the scales filled in by a psychiatrist were statistically significantly greater (p < 0.001) in the groups of patients receiving PPT: in group 2 (ΔMADRS24-0=-20.2±6.57; ΔHAM-A24-0 =-13.2±5.68) and group 3 (ΔMADRS24-0=-17.8±4.73; ΔHAM-A24-0=-13.4±4.41), compared with group 1 (ΔMADRS24-0=-5.42±7.14; ΔHAM-A24-0=-4.58±6.80). There were no significant differences between the groups according to the PHQ-9 depression questionnaire (respectively, in group 1, ΔPHQ-924-0=-4.89±4.87; in group 2, ΔPHQ-924-0 =-6.73±4.97; in group 3, ΔPHQ-924-0=-7.26±5.58), despite a greater decrease in the severity of depression in groups with PPT. According to a semi-structured interview with a psychiatrist and projective experimental psychological techniques, the proportion of patients without depression 24 weeks after the start of therapy was significantly higher in the groups receiving PPT: 84.3% in group 2, 100% in group 3, and 16.3% in group 1.
Conclusion: In patients with moderate/high RA activity and comorbid depression, OKZ without PPT can lead to a decrease in severity of depression in 51% of patients with RA, with complete regression of depressive symptoms observed in 16.3% of patients, mainly those with minor depression. OKZ therapy without PPT also reduces the severity of anxiety, but does not eliminate it completely. The combination of OKZ and PPT is optimal for achieving complete regression of depression and anxiety in this category of RA patients.
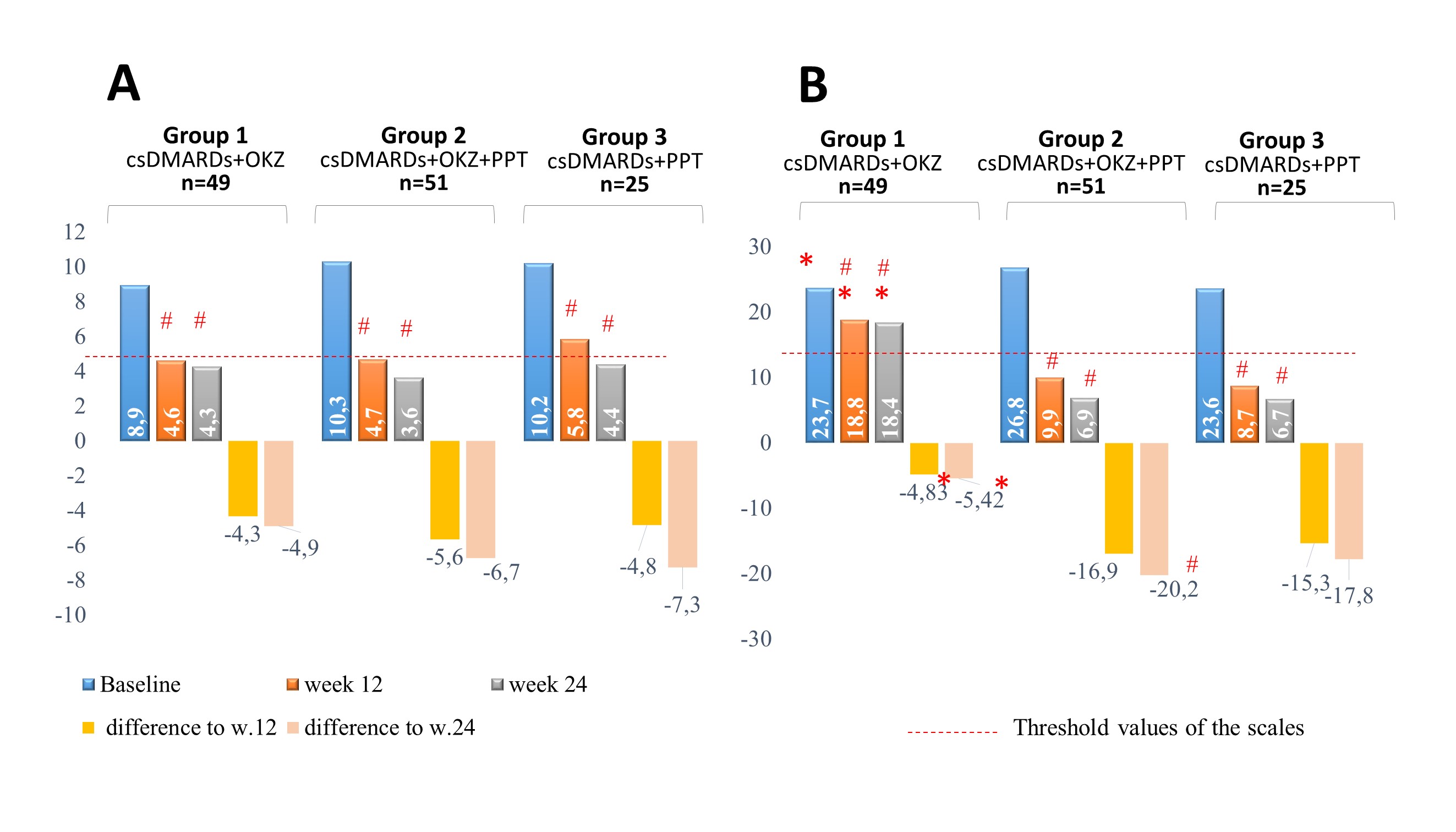 Figure 1. The dynamics of depression severity depending on therapy
Figure 1. The dynamics of depression severity depending on therapy
Note: A, The dynamics of depression severity according to the PHQ-9 self-questionnaire, score
B, The dynamics of depression severity according to the MADRS scale, score; * р < 0,05 comparison between groups; # p < 0,05 comparison between baseline and weeks 12/24 in the group; csDMARDs - conventional synthetic disease modifying antirheumatic drugs; OKZ – olokizumab: PPT - psychopharmacotherapy
.jpg) Figure 2. The dynamics of anxiety severity and the proportion of patients without anxiety according to the HAM-A scale by week 24
Figure 2. The dynamics of anxiety severity and the proportion of patients without anxiety according to the HAM-A scale by week 24
Note: A, The dynamics of anxiety severity on the Hamilton scale, score; B, The proportion of patients with clinically significant anxiety on the Hamilton scale; * р < 0,05 comparison between groups; # p < 0,05 comparison between baseline and weeks 12/24 in the group; csDMARDs - conventional synthetic disease modifying antirheumatic drugs; OKZ – olokizumab: PPT - psychopharmacotherapy
.jpg) Figure 3. The dynamics of the incidence of major/ minor depression according to DSM-5 criteria depending on therapy
Figure 3. The dynamics of the incidence of major/ minor depression according to DSM-5 criteria depending on therapy
Note: csDMARDs – conventional synthetic disease modifying antirheumatic drugs; OKZ – olokizumab: PPT – psychopharmacotherapy; * р < 0,05 (comparison between baseline and week.24 in Groups); # р < 0,001 (comparison between Group 1 and Groups 2-3)
To cite this abstract in AMA style:
Lisitsyna T, Abramkin A, Veltishchev D, Kuzkina S, Borisova A, Ignatiev V, Samsonov M, Nasonov E. Efficacy of Olokizumab in Treating Comorbid Depression in Patients with Rheumatoid Arthritis: Results of a Single-Center Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-of-olokizumab-in-treating-comorbid-depression-in-patients-with-rheumatoid-arthritis-results-of-a-single-center-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-olokizumab-in-treating-comorbid-depression-in-patients-with-rheumatoid-arthritis-results-of-a-single-center-randomized-controlled-trial/
