Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Distinguishing lupus hepatitis (LH) from autoimmune hepatitis (AIH) in SLE is clinically challenging but critical for guiding treatment duration. The mechanisms differ; AIH results from a T-cell-mediated immune response to liver autoantigens, while LH likely results from hepatic vasculitis, complement activation, and rarely, microvascular occlusive disease. On biopsy, AIH is characterized by lymphoplasmacytic interface hepatitis and hepatocyte rosettes; LH presents with nonspecific mild lobular or periportal inflammation. Systematic histopathologic studies differentiating AIH and LH in pediatric SLE are lacking.
Methods: With IRB approval, we queried our EMR (1/1/12–3/1/25) for pediatric SLE patients who underwent liver biopsy. Clinical, laboratory and histopathology data were abstracted. Disease activity at biopsy was assessed using the SLEDAI-2K.
Results: During the study period, of the 452 patients with SLE, 12 (2.7%) underwent liver biopsy. In three, liver disease predated SLE diagnosis; biopsies (median six months prior to diagnosis; range 6–32) revealed two AIH and one Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) later diagnosed as possible AIH on repeat biopsy. In the other nine patients, biopsies occurred at a median of one month post diagnosis with SLE (range 0-37). All were performed at least in part to exclude AIH. One patient diagnosed with AIH before SLE was F-actin IgG positive; the other tested negative for F-actin and anti-LKM. Six out of the nine patients who had negative biopsy findings for AIH were positive for either F-actin, anti-Smooth muscle IgG, or anti-LKM Ab. These patients with positive AIH-associated antibodies but negative biopsy had been pre-treated for a median of one week (range three days-seven months). One patient had three serial liver biopsies, one prior to diagnosis and two after to evaluate for AIH. Peak AST was 211 U/L (range 50-1138) and ALT was 206 U/L (107-1111). Of the nine patients with liver biopsies after diagnosis of SLE, one had AIH, one had MASLD, three had possible drug-related injury (secondary to antibiotics, sertraline, and acetaminophen), and four had nonspecific findings postulated to be SLE-related. Among the four patients with nonspecific findings, all showed mild portal inflammation with infiltrates including plasma cells, lymphocytes, and/or neutrophils. One had mild focal venulitis, one had grade 1 fibrosis, and one had mild hepatocyte ballooning. All had preserved synthetic function. Of the patients with AIH, two were already on azathioprine and prednisone (15–30 mg) at the time of biopsy and remained on the same regimen; one was initiated on azathioprine and prednisone (2 mg/kg), and another with concurrent lupus nephritis and AIH was started on mycophenolate. Nonspecific biopsy findings did not alter management. At median follow-up of six years (range 0.2–9), none had persistent clinically evident liver disease.
Conclusion: Despite a cohort with active SLE with multi-organ involvement, lupus-related liver manifestations were transient and self-limited. Liver biopsy remains crucial in diagnosis of comorbid AIH since AIH-related autoantibodies were variably detected and may be unreliable in active SLE.
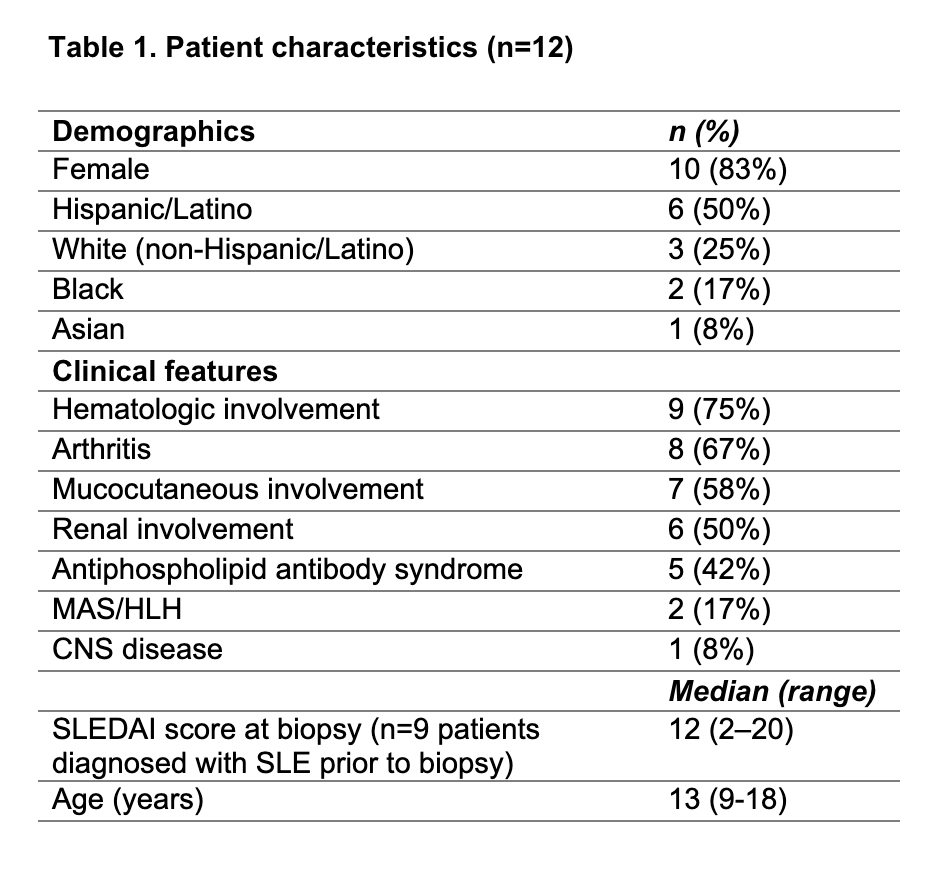 Table 1: Patient characteristics
Table 1: Patient characteristics
To cite this abstract in AMA style:
Rae M, Gist D, Patel K, Mysore K, Ramirez A, De Guzman M, Muscal E. Liver Biopsy Findings in Pediatric SLE: A Large Tertiary Center Experience [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/liver-biopsy-findings-in-pediatric-sle-a-large-tertiary-center-experience/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/liver-biopsy-findings-in-pediatric-sle-a-large-tertiary-center-experience/
