Session Information
Date: Monday, October 27, 2025
Title: (1248–1271) Patient Outcomes, Preferences, & Attitudes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Clinical trials exploring the safety and efficacy of chimeric antigen receptor (CAR) T-cell therapy for lupus are growing. Challenges linked to recruitment for CAR T-cell clinical trials are likely multifactorial and may include barriers at the healthcare system, provider, and patient level. The aim of this study was to elicit qualitative feedback from people living with systemic lupus erythematosus (SLE) who have the greatest burden of disease to understand attitudes, perceptions, and behaviors related to CAR T-cell therapy clinical trials, specifically the Fate Therapeutics FT819 product.
Methods: A focus group was conducted among people with SLE recruited via the Lupus Foundation of America’s Research Accelerated by You (RAY) Registry. Researchers queried RAY for individuals thought to meet inclusion criteria and provided an online screener. Of 205 respondents, 23 were shortlisted based on response timing (5 business days) and alignment with a priori inclusion/exclusion criteria. Upon follow-up contact, 6 expressed interest and consented. Five attended a 2-hour virtual focus group in December 2025 via Zoom. Following a slide presentation describing FT819 therapy as an off-the-shelf (uniquely available on-demand) cell therapy with reduced patient burden such as hospitalization requirements, semi-structured questions covered: (1) background and journey to diagnosis and care, (2) general awareness and perceptions of cell therapy, (3) perceptions of FT819 therapy administration, (4) potential benefits and risks of receiving FT819 therapy within a clinical trial, and (5) perceptions and acceptability of follow-up and monitoring. The focus group was audio-video recorded, transcribed, and analyzed in Dedoose using content analysis methodology.
Results: Participants included 5 Black/African American female adults, one of whom also identified as Hispanic. All had active SLE affecting one or more major organ systems (Table 1). Results emerged across 3 categories: (1) Initial perceptions of cell therapy, (2) perceptions of FT819 therapy including benefits and concerns (Tables 2 and 3), and (3) willingness to participate in an FT819 trial and expected outcomes. Initial perceptions indicated participants were familiar with and optimistic about cell therapy but also had concerns. Regarding FT819 therapy, participants perceived benefits such as improved quality of life, potential for remission, and a relatively short hospital stay. Participants also expressed concerns about potential adverse reactions or side effects, chemotherapy or mycophenolate requirements, long-term effectiveness, time and travel, hospital care and quality, caregiver needs, and medication cost and coverage (upon FDA approval). Participants also commented on willingness to participate in an FT819 trial, definition of successful treatment, and required duration of clinical effect.
Conclusion: Clinical trials in the U.S. have often failed to include representative samples, leading to a lack of generalizability. This study identified barriers and facilitators to cell therapy trial participation among a purposive sample. The findings can guide activities such as patient engagement, recruitment, and site selection.
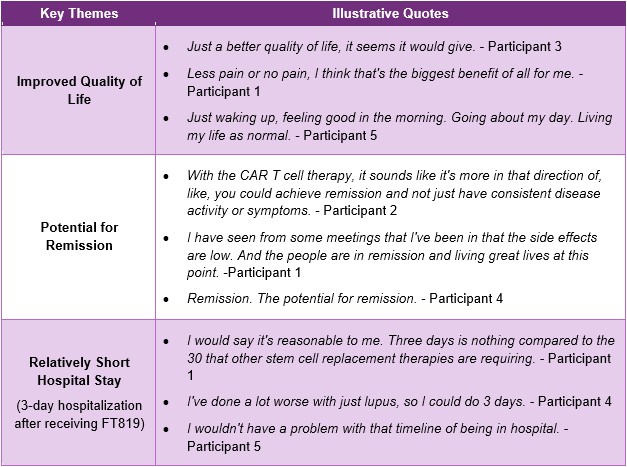 Table 1. Participant Demographics
Table 1. Participant Demographics
.jpg) Table 2. Perceived Benefits of FT819 Therapy
Table 2. Perceived Benefits of FT819 Therapy
.jpg) Table 3. Concerns about FT819 Therapy
Table 3. Concerns about FT819 Therapy
To cite this abstract in AMA style:
Buie J, Agyemang S, Henry A, BitMansour A, Sandhu V, Zack D, Fisher M. Increasing Participation in the FT819 Cell Therapy Trial Amongst People Living with Lupus: A Focus Group Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/increasing-participation-in-the-ft819-cell-therapy-trial-amongst-people-living-with-lupus-a-focus-group-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-participation-in-the-ft819-cell-therapy-trial-amongst-people-living-with-lupus-a-focus-group-study/
