Session Information
Date: Monday, October 27, 2025
Title: (1221–1247) Pain in Rheumatic Disease Including Fibromyalgia Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Diagnosing juvenile fibromyalgia (JFM) in youth with chronic musculoskeletal (MSK) pain is challenging in the absence of a validated diagnostic tool. The Pain and Symptom Assessment Tool (PSAT) was developed from modifications to the ACR criteria for adult FM (2010) to aid in JFM diagnosis. This ongoing study aims to validate the PSAT as a diagnostic tool by demonstrating how measured differences in pain distribution and accompanying symptoms distinguish JFM from other causes of chronic MSK pain such as JIA or more localized painful disorders.
Methods: This multicenter, cross-sectional observational study enrolled English- and Spanish-speaking adolescents (age range: 11 – 17) with MSK pain of ≥3 months’ duration and a clinical diagnosis of JFM, JIA, or localized MSK pain. All participants completed the PSAT, which included both assessment of pain location using the Michigan Body Map© (MBM) and a Symptom Severity Index (SSI) comprised of an inventory of associated symptoms with severity indices for fatigue, nonrestorative sleep, and impaired memory or concentration. One-way ANOVA was performed to look for differences in MBM totals and SSI between diagnostic groups, and post hoc analysis was carried out with Tukey’s HSD test to determine the significance of between-group differences. Yunus and Masi’s criteria for primary JFM (1985), which include ≥5 tender points on physical exam, were used by study staff as a diagnostic standard with which to evaluate all participants for clinical features consistent with JFM.
Results: A total of 158 participants were enrolled across 5 clinical sites. Participant diagnoses and characteristics are detailed in Figure 1. The average pain intensity rating among study participants was 5.7/10 (sd = 2.2), and 77/158 (49%) participants had ≥5 tender points on physical exam. Yunus and Masi’s criteria were fulfilled by 47/60 (78%) participants with a clinical diagnosis of JFM and one participant with JIA. No participants with localized pain met these criteria. MBM totals and SSI both differed between diagnostic groups with F(2, 155) = 43.43 (p < 0.001, 95% CI [0.26, 1.00]) and F(2, 155) = 25.79 (p < 0.001, 95% CI [0.15, 1.00]), respectively. Post hoc analysis revealed significantly higher MBM totals and SSI among participants with JFM when compared to those with either localized MSK pain or JIA (p < 0.001). Figures 2 and 3 illustrate the distribution of MBM totals and SSI across participant diagnoses.
Conclusion: Analysis of these results from PSAT administration to adolescents with chronic MSK pain shows clear differences in measured pain distribution and symptom severity between those participants diagnosed clinically with JFM and those with other chronically painful MSK conditions. As recruitment for this study is ongoing, sensitivity and specificity of the PSAT in comparison with Yunus and Masi’s criteria for the diagnosis of JFM will be determined following completion of further data collection and analysis. However, these preliminary results support the utility of the PSAT in distinguishing JFM from other causes of chronic MSK pain in pediatric patients, highlighting a valuable tool to improve the consistency of case definitions in research and ease diagnostic uncertainty in clinical care.
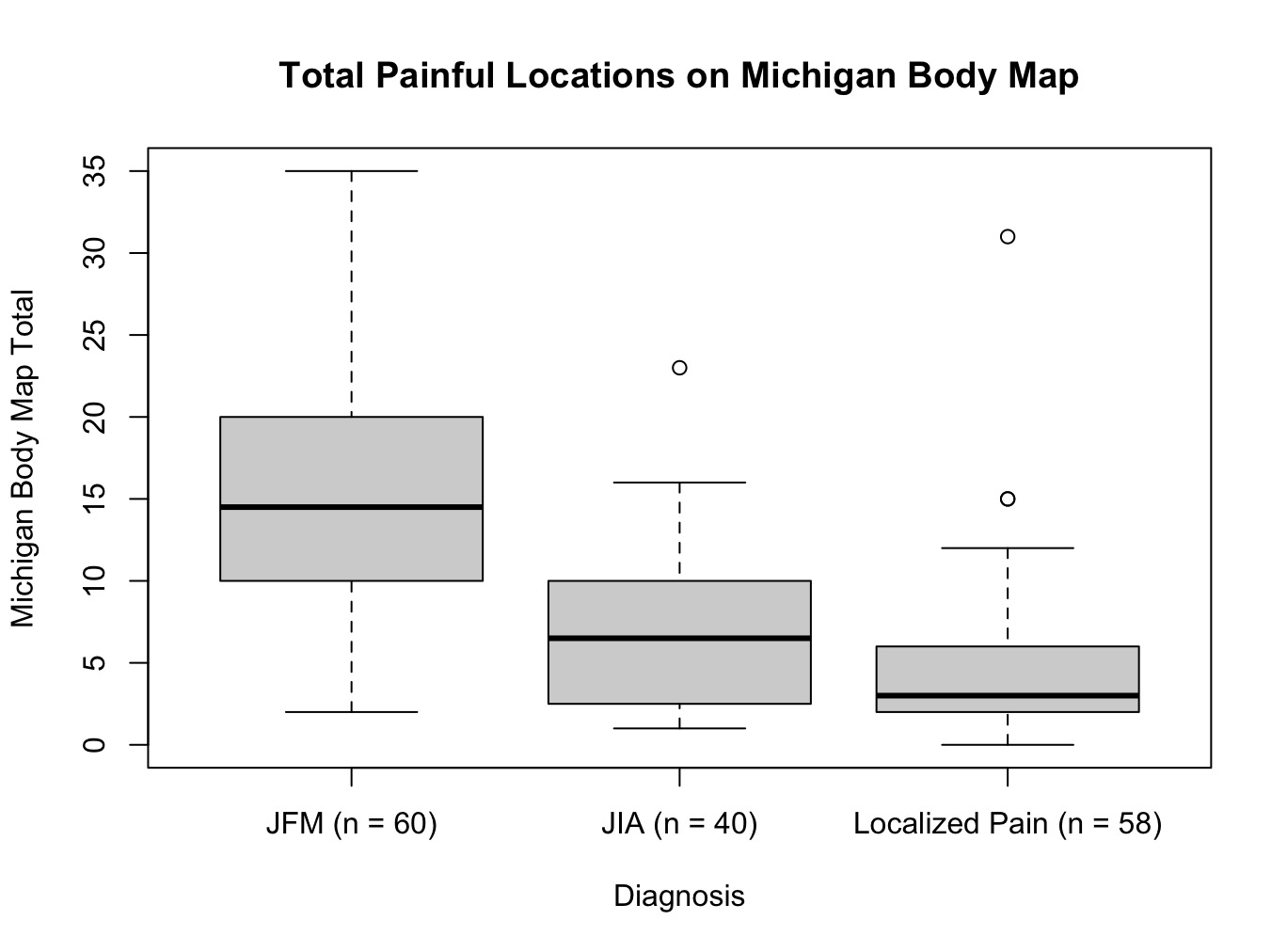 Figure 1: A table providing demographic and clinical characteristics of enrolled participants broken down by diagnosis. Included are age, gender, race, ethnicity, language of study completion, pain intensity, MBM total, SSI, tender point exam findings, and fulfillment of Yunus and Masi’s critera.
Figure 1: A table providing demographic and clinical characteristics of enrolled participants broken down by diagnosis. Included are age, gender, race, ethnicity, language of study completion, pain intensity, MBM total, SSI, tender point exam findings, and fulfillment of Yunus and Masi’s critera.
.jpg) Figure 2: A box plot of total painful locations on Michigan Body Map (MBM) broken down by participant diagnosis. Center bars represent the median. Upper and lower limits of boxes are interquartile ranges. The limits of the whiskers are the full range with significant outliers excluded and plotted separately.
Figure 2: A box plot of total painful locations on Michigan Body Map (MBM) broken down by participant diagnosis. Center bars represent the median. Upper and lower limits of boxes are interquartile ranges. The limits of the whiskers are the full range with significant outliers excluded and plotted separately.
.jpg) Figure 3: A box plot of participants’ Symptom Severity Index (SSI) broken down by diagnosis. Center bars represent the median. Upper and lower limits of boxes are interquartile ranges. The limits of the whiskers are the full range.
Figure 3: A box plot of participants’ Symptom Severity Index (SSI) broken down by diagnosis. Center bars represent the median. Upper and lower limits of boxes are interquartile ranges. The limits of the whiskers are the full range.
To cite this abstract in AMA style:
Schocken D, Connelly M, Gmuca S, Ramirez A, Riordan M, Ting T, Kashikar-Zuck S, Weiss J. Preliminary Findings in the Validation of the Modified Pain and Symptom Assessment Tool in Juvenile Fibromyalgia Syndrome [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/preliminary-findings-in-the-validation-of-the-modified-pain-and-symptom-assessment-tool-in-juvenile-fibromyalgia-syndrome/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-findings-in-the-validation-of-the-modified-pain-and-symptom-assessment-tool-in-juvenile-fibromyalgia-syndrome/
