Session Information
Date: Monday, October 27, 2025
Title: (1191–1220) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-Signal Recognition Particle (Anti-SRP) Myositis is a form of Polymyositis. Available consensus guidelines recommend initial combination therapy of high dose corticosteroids and Methotrexate and addition of Rituximab (RTX) within 6 months of starting therapy.
Methods: Objectives:1. Comparison of non-rituximab vs Rituximab therapy in treatment naïveAnti-SRP Myositis patients.2. Comparison of Rituximab in Treatment refractory vs Treatment naïveanti-SRP Myositis patients.Methods:It is a Single centre retrospective study. Patients satisfying EULAR/ACRclassification criteria for Idiopathic Inflammatory Myopathy AND anti-SRPantibody in moderate to high titer were defined as cases. Patients withconcomitant presence of any other Myositis specific antibody (MSA) inmoderate to high titer were excluded. Complete remission (CR) was defined asMMT increase of≥ 20% 𝑓𝑟𝑜𝑚 𝑏𝑎𝑠𝑒𝑙𝑖𝑛𝑒 𝑖𝑛 𝑀𝑎𝑛𝑢𝑎𝑙 𝑀𝑢𝑠𝑐𝑙𝑒 𝑡𝑒𝑠𝑡𝑖𝑛𝑔 (𝑀𝑀𝑇 ― 8) ANDnormalisation of CPK while partial remission was defined as≥ 20% 𝑖𝑛𝑐𝑟𝑒𝑎𝑠𝑒 𝑖𝑛 𝑀𝑀𝑇 ― 8 but elevated CPK ( >2 times of ULN). Patientwith ≥ 20% worsening in MMT from highest value and/or > 3 times increasein CPK from lowest value while on standard of care therapy were defined asrefractory cases.
Results: A total of 13 patients of SRP Myositis were included. Out of 13 cases-8 casesinitially received Immunosuppressants other than Rituximab (defined as initialnon-rituximab therapy group) while 5 cases initially received Rituximab(defined as initial Rituximab Therapy group). In initial non-rituximab therapygroup, 3 received Methotrexate, 2 received monthly Cyclophosphamidetherapy for 6 months, 2 received Mycophenolate Mofetil and 1 receivedAzathioprine. All 8 cases relapsed after Complete or partial remission duringfollow-up and met the criteria of refractory disease. Refractory patientsreceived Inj. Rituximab 1 gm two doses. In initial Rituximab therapy group casesreceived Rituximab 1 gm two doses and did not receive any other conventionalDMARD (C-DMARDs).
Conclusion: 1. Complete remission (CR) at 6 months is higher in initial rituximab therapygroup in treatment naïve anti-SRP Myositis patients. However, bothgroups showed similar improvement in MMT-8, reduction in CPK, andcorticosteroid dose reduction at 6 months after starting therapy.2. RTX therapy is equally effective at 6 months in treatment refractory andtreatment naïve patients in terms of improvement in MMT and reductionin CPK. Treatment-naïve patients had significantly greater corticosteroidneeds throughout the treatment period.
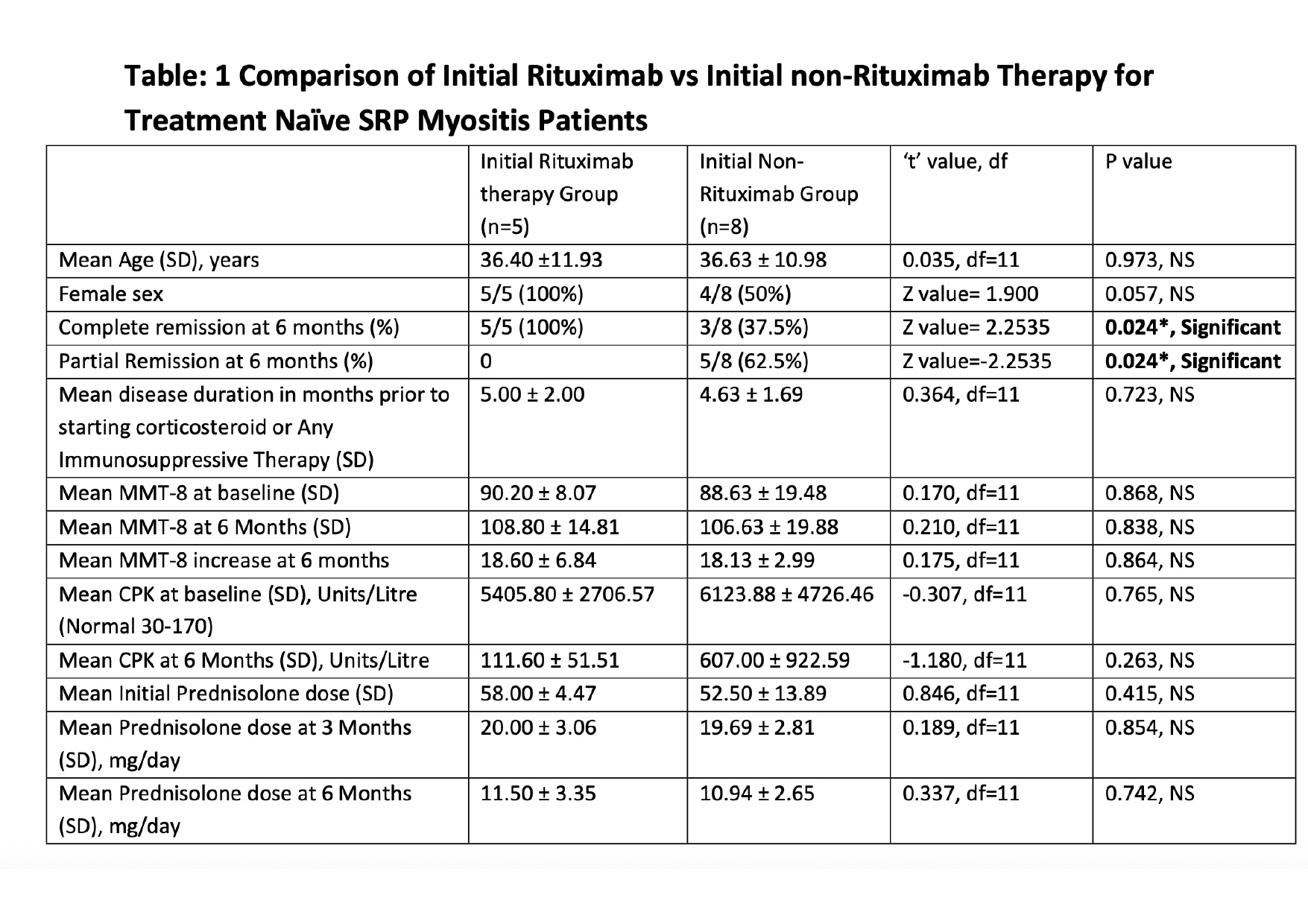 Table: 1 Comparison of Initial Rituximab vs Initial non-Rituximab Therapy for
Table: 1 Comparison of Initial Rituximab vs Initial non-Rituximab Therapy for
Treatment Naïve SRP Myositis Patients
.jpg) Table 2: Comparison of Rituximab Therapy in Treatment refractory and Treatment naive anti-SRP myositis patients.
Table 2: Comparison of Rituximab Therapy in Treatment refractory and Treatment naive anti-SRP myositis patients.
To cite this abstract in AMA style:
haque i, Ghosh P. Rituximab in Treatment Refractory vs Treatment Naive Anti-Signal Recognition Particle (SRP) Myositis – A Case Series [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rituximab-in-treatment-refractory-vs-treatment-naive-anti-signal-recognition-particle-srp-myositis-a-case-series/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-in-treatment-refractory-vs-treatment-naive-anti-signal-recognition-particle-srp-myositis-a-case-series/
