Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To estimate the incidence and identify associated factors of azathioprine (AZA)-induced leukopenia.
Methods: A retrospective cohort study was conducted on 1,062 adult patients with non-malignant diseases who received azathioprine for the first time between 2014 and 2023 at Siriraj Hospital, Thailand. Inclusion criteria required patients to have a baseline complete blood count (CBC) within eight weeks before starting AZA and a follow-up CBC performed two weeks after treatment. Patients were excluded if they had an abnormal CBC, defined as a white blood cell (WBC) count < 3,000 cell/μl, a neutrophil count < 1,500 cell/μl (neutropenia), or a lymphocyte count < 1,500 cell/μl. Patients with active infections or a history of splenectomy were also excluded. Clinical and laboratory parameters were collected from a medical database during the first year of azathioprine treatment. Follow-up CBC were analyzed to identify cases of AZA-induced leukopenia, defined as a WBC count < 3,000 cell/μl and/or neutropenia. Hazard ratios for factors associated with AZA-induced hematologic abnormalities was analyzed using Cox regression.
Results: Among the cohort, SLE was the most common underlying condition, affecting 274 patients (25.80%). The overall incidence of AZA-induced leukopenia was 6.21%, with an incidence rate of 5.28 per 1,000 person-months (95% CI: 4.15–6.73). Notably, SLE patients had a significantly higher incidence of 12.04% and an incidence rate of 10.60 per 1,000 person-months (95% CI: 7.54–14.91), P < 0.05 compared to non-SLE populations. Incidence and incidence rates of each lineage of WBC abnormalities with different cut point were shown in Table 1. SLE was a significant risk factor for AZA-induced leukopenia (HR = 3.001, P < 0.001), as well as for leukopenia, neutropenia, and lymphopenia. Other predictors included AZA dose >100 mg/day, associated with higher risk of leukopenia (HR = 9.609, P < 0.001) and neutropenia (HR = 6.719, P < 0.001). Concurrent allopurinol use conferred the highest risk for leukopenia (HR = 17.007, P < 0.001). Lower baseline WBC and neutrophil counts were linked to higher risk of AZA-induced leukopenia and neutropenia, as shown in Table 2. SLE remained an independent risk factor after adjusting for baseline leukocyte counts and AZA dose. Additionally, AZA-induced leukopenia incidence did not significantly differ between patients with normal pharmacogenetic profiles for Thiopurine S-methyltransferase (TPMT), Inosine triphosphate pyrophosphatase 94C >A (ITPA 94C >A), and Nudix hydrolase 15C415T (NUDT15 C415T) and those without testing.
Conclusion: Given the notably high incidence of AZA-induced leukopenia in SLE patients, regular CBC monitoring and AZA dose adjustment are essential to prevent its occurrence, even in those with normal baseline CBC at the start of treatment. Additionally, patients receiving allopurinol concurrently, an AZA dose over 100 mg/day, or those with low baseline WBC and neutrophil counts should be monitored closely. Relying solely on pharmacogenetic testing (including TPMT, ITPA, and NUDT15) may not be sufficient to prevent AZA-induced leukopenia.
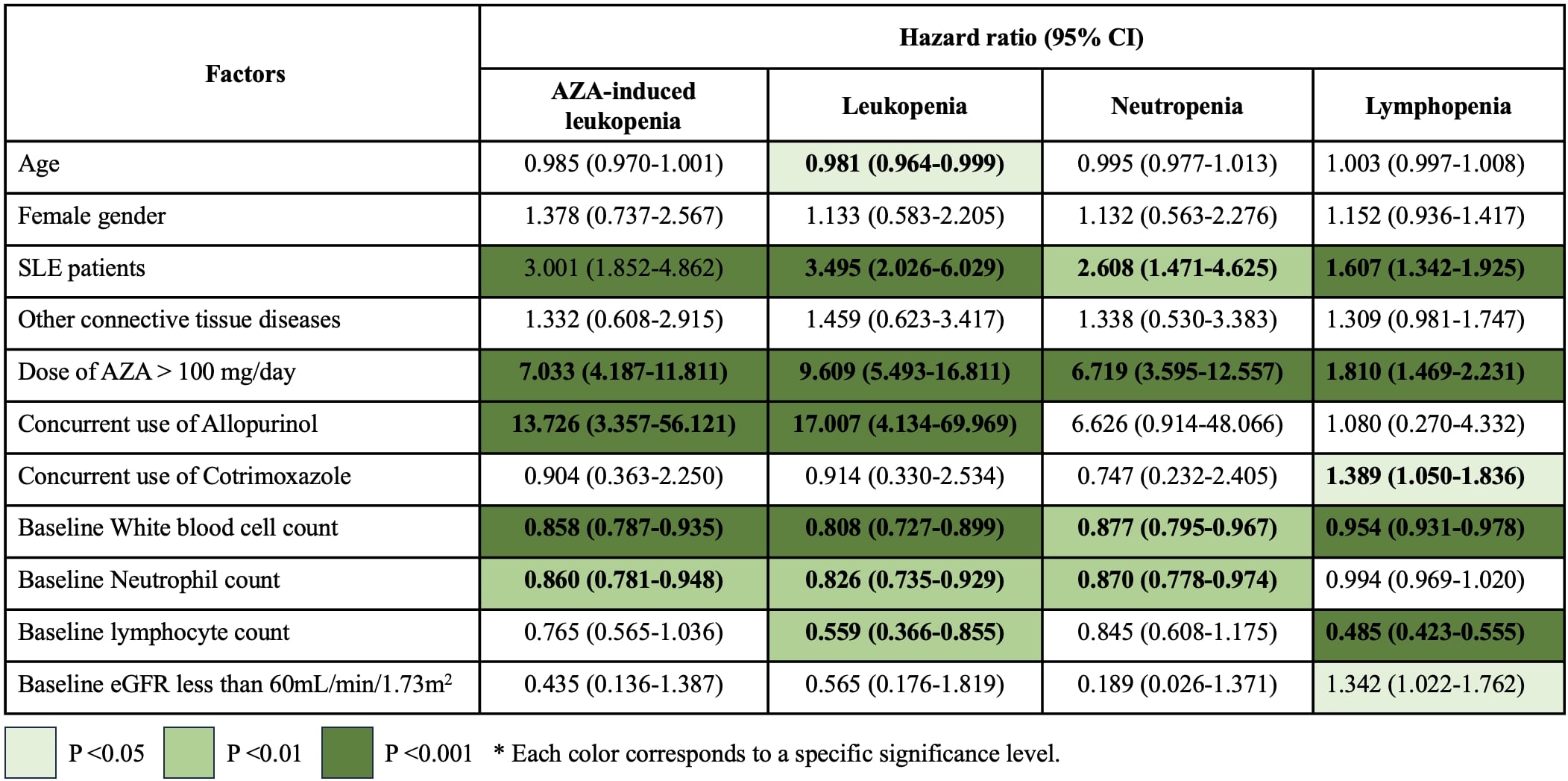 Table 1: Incidence and incidence rates of azathioprine-induced hematologic abnormalities in the overall cohort and the SLE subgroup.
Table 1: Incidence and incidence rates of azathioprine-induced hematologic abnormalities in the overall cohort and the SLE subgroup.
.jpg) Table 2: Hazard ratios with 95% confidence intervals for factors associated with azathioprine-induced hematologic abnormalities, analyzed using Cox regression. Statistically significant associations are highlighted by color intensity based on P values. AZA-induced leukopenia was defined as a white blood cell (WBC) count < 3,000 cells/μL and/or neutrophil count < 1,500 cells/μL. Individual abnormalities were classified as follows: leukopenia (WBC < 3,000 cells/μL), neutropenia (neutrophil count < 1,500 cells/μL), and lymphopenia (lymphocyte count < 1,500 cells/μL).
Table 2: Hazard ratios with 95% confidence intervals for factors associated with azathioprine-induced hematologic abnormalities, analyzed using Cox regression. Statistically significant associations are highlighted by color intensity based on P values. AZA-induced leukopenia was defined as a white blood cell (WBC) count < 3,000 cells/μL and/or neutrophil count < 1,500 cells/μL. Individual abnormalities were classified as follows: leukopenia (WBC < 3,000 cells/μL), neutropenia (neutrophil count < 1,500 cells/μL), and lymphopenia (lymphocyte count < 1,500 cells/μL).
To cite this abstract in AMA style:
Buadee S, Wilasrusmee K, Kamonsumlitpon T, Charuvanij S, Karoopongse E, Chiowchanwisawakit P. Increased Risk of Azathioprine-Induced Leukopenia in Patients with Systemic Lupus Erythematosus: A 10-Year Retrospective Cohort Study at a Quaternary Care Hospital in Thailand [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/increased-risk-of-azathioprine-induced-leukopenia-in-patients-with-systemic-lupus-erythematosus-a-10-year-retrospective-cohort-study-at-a-quaternary-care-hospital-in-thailand/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-risk-of-azathioprine-induced-leukopenia-in-patients-with-systemic-lupus-erythematosus-a-10-year-retrospective-cohort-study-at-a-quaternary-care-hospital-in-thailand/
