Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of an increasing number of malignancies. Because patients with pre-existing autoimmune disease have been excluded from clinical trials of ICIs, there is a paucity of data regarding outcomes in patients with pre-existing rheumatoid arthritis (RA). The aim of our study was to describe rheumatologic and oncologic outcomes of patients with a pre-existing diagnosis of RA who received ICIs for treatment of their malignancy.
Methods: We conducted a cohort study of previously collected data at a single center. We used TriNetX to broadly identify patients receiving an immune checkpoint inhibitor and a disease-modifying antirheumatic drug (DMARD) from January 1st, 2010 to September 19th, 2023. Electronic medical records were abstracted to identify patients with pre-existing RA, patient demographics, laboratory investigations, type of malignancy, type of ICI administered, and flares of RA. Response Evaluation Criteria in Solid Tumors (RECIST) at 6 months was performed by a radiologist to assess for malignancy progression.
Results: We identified 22 patients who had pre-existing RA and received both an ICI and a DMARD. The mean patient age was 70.4 years old and on average patients had been diagnosed with RA for 6 years. There was a history of tobacco use in 77.3% (17/22) of patients. The most common malignancy was non-small cell lung cancer in 31.8% (7/22). Pembrolizumab was the most common ICI with 77.3% (17/22) of patients receiving it. Methotrexate had been used by 81.8% (18/22) of patients, hydroxychloroquine by 86.4% (19/22), and sulfasalazine by 18.2% (4/22). Rheumatoid factor was positive in 40.1% (9/19) and anti-CCP was positive in 50% (8/16). 45% (10) of patients experienced a flare of their RA. Of the 10 patients who had an RA flare, 9 flared by 6 months following ICI initiation, and 8 after one cycle of ICI. RECIST at 6 months after ICI initiation revealed progressive disease in 59.1% (13/22), stable disease in 22.7% (5/22), partial response in 9.1% (2/22), and complete response in 9.1% (2/22).
Conclusion: Approximately half of patients with pre-existing RA treated with ICIs had flares of RA, typically after one dose of ICI administration. Eight of the ten patients who had RA flares flared within six months of ICI initiation. The average number of cycles of ICI administrations prior to flare was 2.7. The majority of patients had been treated with methotrexate and hydroxychloroquine. The rate of progressive disease at 6 months was high, though reflective of the diversity of malignancies included. It is important to be aware of a high flare rate in patients with RA who are undergoing ICI therapy. It would be prudent to increase the frequency of rheumatologic follow up in this patient population in order to quickly diagnose and treat RA flares secondary to oncologic therapy. As most patients who experience an RA flare following ICI initiation do so following the first ICI administration, it may be useful to schedule follow up within a month of initiation. Future efforts to delineate the effects of DMARD therapy management decisions when initiating ICI therapy on both oncologic and rheumatic outcomes are planned.
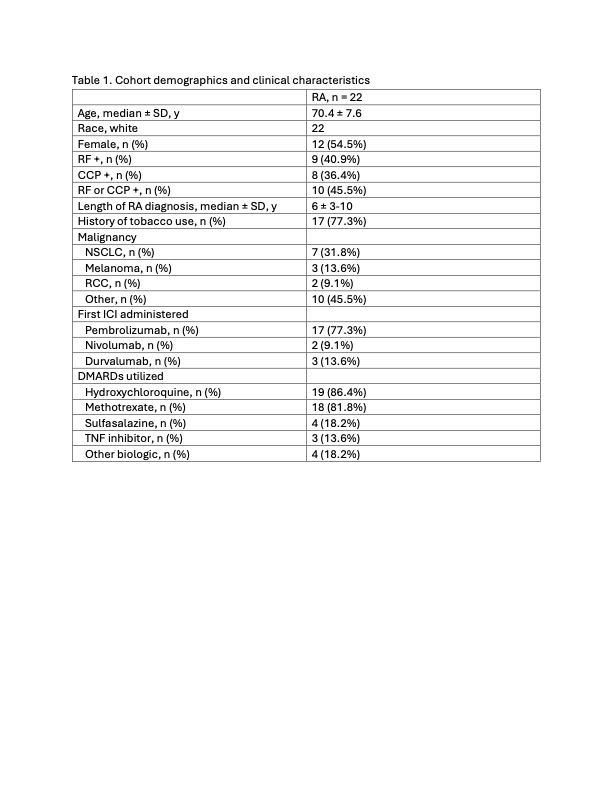 Table 1. Cohort demographics and clinical characteristics
Table 1. Cohort demographics and clinical characteristics
.jpg) Table 2. Tumor outcomes and rheumatoid arthritis flare rates
Table 2. Tumor outcomes and rheumatoid arthritis flare rates
.jpg) Figure 1. Flow diagram describing the stepwise approach utilized to determine patient cohort
Figure 1. Flow diagram describing the stepwise approach utilized to determine patient cohort
To cite this abstract in AMA style:
Walsh M, Zhang J, Abuqayas B, Jain S, Zakharia Y, Lenert A, Strouse J. Tumor outcomes and arthritis flares in patients with pre-existing rheumatoid arthritis receiving immune checkpoint inhibitor therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/tumor-outcomes-and-arthritis-flares-in-patients-with-pre-existing-rheumatoid-arthritis-receiving-immune-checkpoint-inhibitor-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tumor-outcomes-and-arthritis-flares-in-patients-with-pre-existing-rheumatoid-arthritis-receiving-immune-checkpoint-inhibitor-therapy/
