Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Inflammatory arthritis (IA)- and polymyalgia rheumatica (PMR)-like syndromes occur in about 6% of patients receiving an immune checkpoint inhibitor (ICI) and can worsen quality of life. Finding the balance between immunosuppression use without affecting the tumor response is critical. Currently, ICI-IA/PMR management is not standardized and is based on expert opinion. It is unknown whether ICI-IA and ICI-PMR can be treated with the same immunosuppressive regimens. We aimed to compare the utilization of steroids and other DMARDs between these two entities.
Methods: Data were extracted from the Rheumatology Adverse Events Due to Immunotherapy Observational Study (RADIOS) registry regarding patients with ICI-IA or ICI-PMR and no preexisting IA who had been prospectively recruited into RADIOS since 2015 at nine sites around the United States. Data was downloaded on 11/6/2024. Steroids were defined as methylprednisolone and prednisone. We excluded hydrocortisone, used for adrenal replacement, and dexamethasone, used for cancer-related symptoms. Cumulative steroid utilization from the time of onset of IA was available for a subset of patients. We analyzed only immunosuppressive DMARDs, such as methotrexate, leflunomide and biologic/targeted synthetic DMARDs, because of potential impact to tumor responses from ICIs. Categorical and continuous data were compared using descriptive statistics. Kaplan Meier curves were used for survival analyses and groups were compared using a log rank test. We measured the time to a prednisone equivalent dosage i. 10 mg/day or ii. 5 mg/day starting from the first steroid dose received at or after the date of the patient’s baseline visit in RADIOS. Multivariable logistic regression was used to compare DMARD utilization in ICI-IA versus ICI-PMR, adjusted for maximum grade (severity), cancer type, the use of combination ICI (anti-CTLA4 plus anti-PD1), recruitment institution and age.
Results: 443 patients were included, n=379 with ICI-IA and n=64 with ICI PMR. 49% were female, with a median age of 66 years (IQR 55,73). Anti PD-1/PDL-1 was used in 265 patients (72%) and combination ICI in 92 (25%). At baseline, 11 ICI-PMR patients (20%) had a peripheral synovitis primarily involving the hand, knee and wrist (Table 1). The median maximum steroid dose prescribed after baseline did not differ between groups (p=0.92) (Table 2). Time to reach a steroid dose of 10 or 5 mg/day from first steroid prescription after baseline was also comparable (p=0.74 and p=0.50 respectively) (Figure 1 A, B). There was no difference in average daily steroid dose between the two groups in the first 6 months after symptoms onset (Table 2, Figure 1 C, D). DMARDs were more prescribed to patients with ICI-IA (43%) than ICI-PMR (23%) (p=0.004). This difference remained after adjusting for confounders in a multivariable logistic regression model (OR= 3.03 [95%CI 1.10, 8.34], p=0.032).
Conclusion: Patients with ICI-IA were more likely to be treated with immunosuppressive DMARDs than patients with ICI-PMR. Treatment with steroids was comparable between the two groups. Identifying more severe phenotypes at diagnosis might help to avoid longer steroid exposure before DMARD initiation.
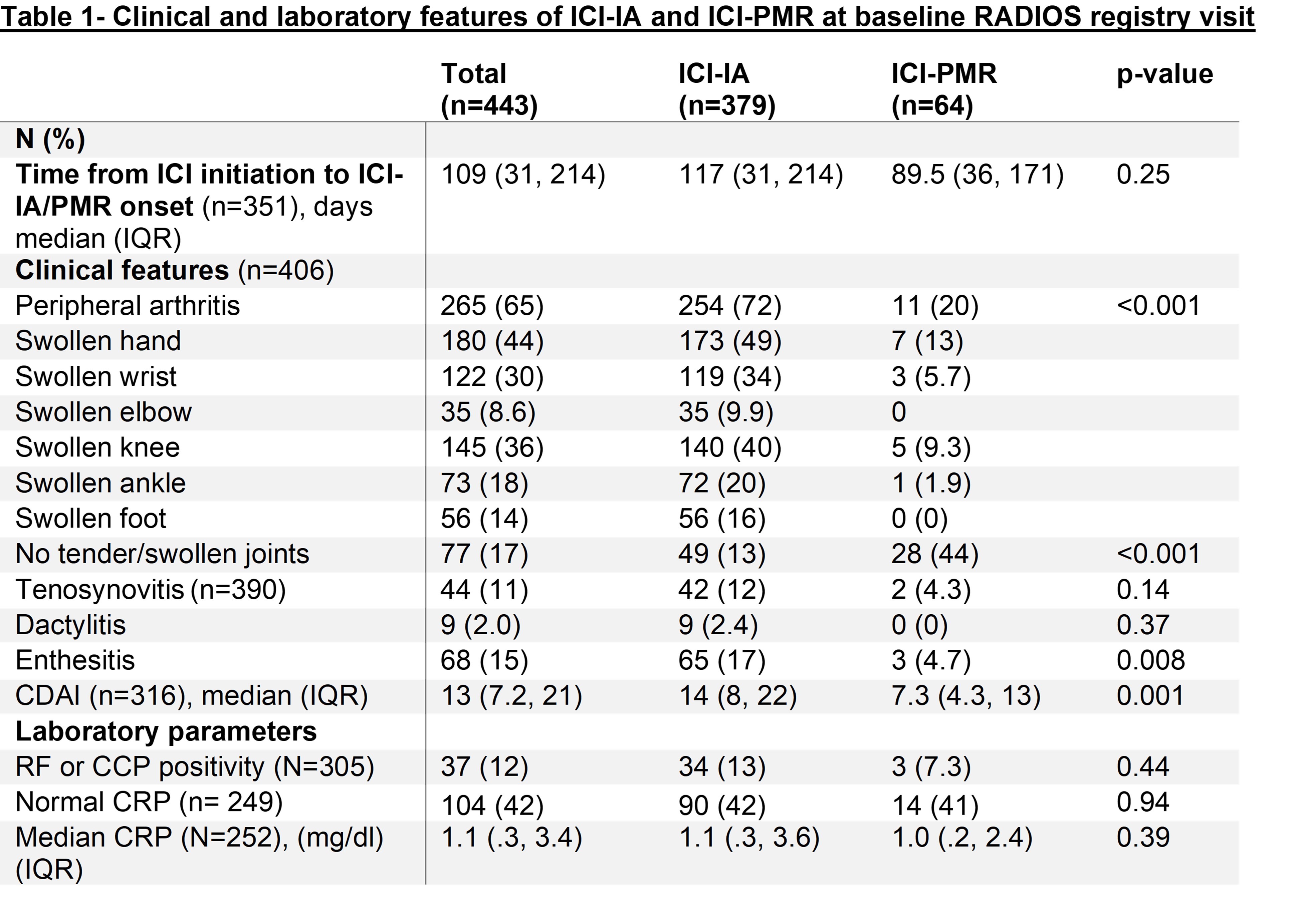 ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, RF : Rheumatoid factor
ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, RF : Rheumatoid factor
.jpg) BL: Baseline RADIOS registry visit, b/ts DMARD: biologic/targeted synthetic Disease Modifying Antirheumatic Drug, csDMARD: conventional synthetic Disease Modifying Antirheumatic Drug, ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, irAE : immune related adverse event. # azathioprine in 2, cytoxan in 2, IVIg in 5, leflunomide in 21 (all in the ICI-IA group), mycophenolate mofetil in 4, sulfasalazine in 13, other in 7 , ## abatacept in 3 (all in the ICI-IA group), rituximab in 3 (all in the ICI-IA group), JAK inhibitor in 5 (all in the ICI-IA group), other in 10.
BL: Baseline RADIOS registry visit, b/ts DMARD: biologic/targeted synthetic Disease Modifying Antirheumatic Drug, csDMARD: conventional synthetic Disease Modifying Antirheumatic Drug, ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, irAE : immune related adverse event. # azathioprine in 2, cytoxan in 2, IVIg in 5, leflunomide in 21 (all in the ICI-IA group), mycophenolate mofetil in 4, sulfasalazine in 13, other in 7 , ## abatacept in 3 (all in the ICI-IA group), rituximab in 3 (all in the ICI-IA group), JAK inhibitor in 5 (all in the ICI-IA group), other in 10.
.jpg) * Baseline = Baseline RADIOS registry visit
* Baseline = Baseline RADIOS registry visit
To cite this abstract in AMA style:
Tison A, Jannat-Khah D, Cappelli L, Bass A. Comparing the immunosuppressant burden in immune checkpoint inhibitor mediated inflammatory arthritis versus polymyalgia rheumatica: results from a prospective multicenter registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparing-the-immunosuppressant-burden-in-immune-checkpoint-inhibitor-mediated-inflammatory-arthritis-versus-polymyalgia-rheumatica-results-from-a-prospective-multicenter-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-the-immunosuppressant-burden-in-immune-checkpoint-inhibitor-mediated-inflammatory-arthritis-versus-polymyalgia-rheumatica-results-from-a-prospective-multicenter-registry/
