Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The broad use of immune checkpoint inhibitors (ICIs) in oncology has led to the emergence of ICI-induced inflammatory arthritis (ICI-IA). The aim of this study was to define ICI-IA phenotypic patterns using unsupervised clustering and latent class analysis (LCA) and determine their association with the need for an immunosuppressive DMARD.
Methods: Data was extracted on 11/6/2024 from the Rheumatology Adverse Events Due to Immunotherapy Observational Study (RADIOS) registry regarding patients with ICI-IA or ICI-polymyalgia rheumatica (PMR) who had been prospectively recruited at nine sites around the United States. Clinical data at the date of registry enrollment was used to group patients. Clinical features involving < 10% of patients were excluded to avoid outliers. We analyzed only immunosuppressive DMARDs (methotrexate, leflunomide, biologic/targeted synthetic DMARDs). Multiple correspondence analysis (MCA) was used to group binary variables followed by a hierarchical classification on the generated coordinates using the Ward method, with Euclidean distances. An LCA was then performed to group patients. The optimal number of classes was determined according to goodness of fit. Classes were compared using descriptive statistics. Stepwise backwards multivariable logistic regression (retaining variables with p ≤0.1) was used to compare DMARD utilization between classes adjusted for maximum ICI-IA/PMR grade, cancer type, the use of combination ICI, recruitment institution and age. The LCA model was then validated in the HSS Registry, an independent single center prospective ICI-IA/PMR registry, and the same statistical methods were applied to compare DMARD utilization between classes.
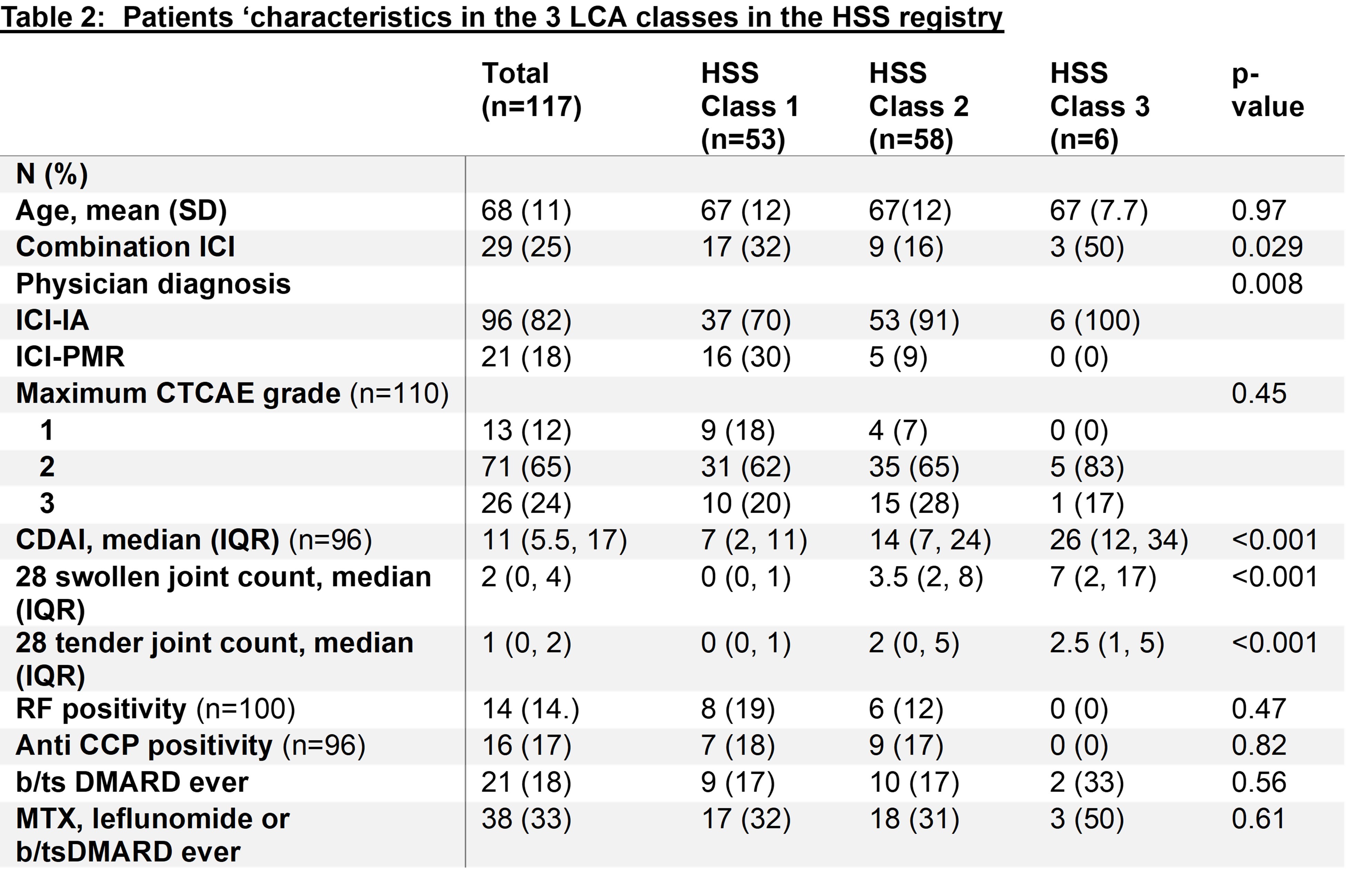
Results: 443 patients with ICI-IA/PMR in the RADIOS registry were included in the cluster analysis. The variables included in the MCA were: swollen hand/wrist/knee/ankle/foot, tenosynovitis and enthesitis. The hierarchical classification identified 3 main groups (figure 1A) and isolated a group of variables, swollen ankle/foot, enthesitis and tenosynovitis, from the others. The LCA identified 3 classes of patients (figure 1B). Class 1 was dominated by rare swollen joints, while swollen hand, wrist and/or knee predominated in Class 2. Swollen ankle and foot reached a probability >80% in class 3, along with a high probability of having other swollen joints. The median CDAI was higher in class 3, as was the 28 swollen joint count (Table 1). The stepwise logistic regression model showed an OR for DMARD use of 3.64 ([95%CI 1.5-9.1] p=0.006) for class 3 compared to class 1. The estimates of the LCA were applied to the validation cohort (n=117) and demonstrated greater use of DMARDs in HSS class 3 compared to class 1 and 2 (Table 2), but this was not statistically significant (OR 3.7 [95%CI 0.5-27], p=0.2), likely due to the small number of patients in class 3 (n=6).
Conclusion: In this large multicenter prospective cohort we identified 3 phenotypic patterns of ICI-IA. One group, characterized by ankle and foot joint swelling and more swollen joints in general, was more likely to require an immunosuppressive DMARD. This information could help stratify patients prognostically at their first rheumatology visit.
.jpg) b/tsDMARD: biologic/targeted synthetic Disease Modifying Antirheumatic Drug, ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, LCA: Latent Class Analysis, MTX: methotrexate, RF: rheumatoid factor.
b/tsDMARD: biologic/targeted synthetic Disease Modifying Antirheumatic Drug, ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, LCA: Latent Class Analysis, MTX: methotrexate, RF: rheumatoid factor.
.jpg) b/tsDMARD: biologic/targeted synthetic Disease Modifying Antirheumatic Drug, ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, LCA: Latent Class Analysis, MTX: methotrexate, RF: rheumatoid factor.
b/tsDMARD: biologic/targeted synthetic Disease Modifying Antirheumatic Drug, ICI-IA: immune checkpoint inhibitor mediated inflammatory arthritis, ICI-PMR: immune checkpoint inhibitor mediated polymyalgia rheumatica, LCA: Latent Class Analysis, MTX: methotrexate, RF: rheumatoid factor.
To cite this abstract in AMA style:
Tison A, Jannat-Khah D, Cappelli L, Bass A. Unsupervised characterization of immune checkpoint inhibitor induced inflammatory arthritis using cluster and latent class analysis: result from a multicenter prospective registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/unsupervised-characterization-of-immune-checkpoint-inhibitor-induced-inflammatory-arthritis-using-cluster-and-latent-class-analysis-result-from-a-multicenter-prospective-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unsupervised-characterization-of-immune-checkpoint-inhibitor-induced-inflammatory-arthritis-using-cluster-and-latent-class-analysis-result-from-a-multicenter-prospective-registry/