Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Randomized controlled trials (RCTs) generate unbiased efficacy estimates and are required for regulatory approval. Understanding the degree to which they include racial, ethnic, sex, and geographical groups is important in understanding their generalizability and in addressing healthcare disparities. This systematic review summarizes the current state of representation in ANCA-associated vasculitis (AAV) and giant cell arteritis (GCA).
Methods: A systematic literature review using PubMed, EMBASE, CINAHL Complete, Europe PMC, ClinicalTrials.gov, and the International Clinical Trials Registry Platform was performed. All pharmacologic phase 2,3, and 4 RCTs in AAV and GCA (2010-2023) were included. This study followed the protocol of a larger systematic review (PROSPERO CRD42023457996), however all RCTs independent of age cut-offs were included in this analysis. Two authors independently reviewed the titles, abstracts, and full texts. Data extraction included demographics, year of publication, trial phase, sponsor category, and location of enrolling sites (continent, country, ZIP+4 for U.S. sites). U.S. enrollment sites were categorized at the county level using Urban-Rural Classification Scheme (small, medium, large metropolitan, and non-metropolitan) and into Medically Underserved Areas (MUA) (yes/no) according to the U.S. Department of Health & Human Services data. Diverse populations were defined as enrolling ≥20% non-White and/or ≥45% female participants based on U.S. Census Data.
Results: A total of 32 AAV RCTs (3954 participants, mean age 58.6 years) and 9 GCA RCTs (773 participants, mean age 71.7) were included. Less than 35% of trials reported race or ethnicity in their results. Among 14 trials that included data on race, the median percentage was highest for white (89.2%) followed by Asian (3.7%) and Black (1.4%) participants. Among 10 trials that included data on ethnicity, the median percentage of Hispanic or Latino participants was 2.0%. Out of 40 trials that reported data on sex, 49.9% were female. The majority of RCTs were multicentric (AAV 87.5%, GCA 88.9%). European centers participated in 78.1% of AAV and 88.9% of GCA RCTs; North American centers participated in 34.4% of AAV and 55% of GCA RCTs. No trials were conducted in Africa (Figure 1). A total of 172 U.S. sites participated in enrollment, of which 120 (69.8%) were located in MUAs. The majority (69.8%) of sites were situated in large metropolitan areas (Figure 2).
Conclusion: Enrollment in AAV and GCA RCTs has been primarily concentrated in Europe, North America, and Australasia. In the U.S., most participating sites are located in large metropolitan areas. The representation of diverse racial and ethnic groups has been notably limited across these trials. Greater efforts are needed to ensure adequate representation in AAV and GCA clinical trials.
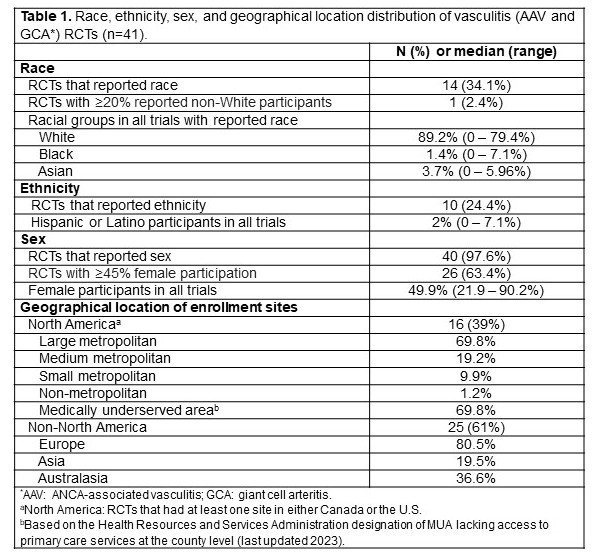 Table 1. Race, ethnicity, sex, and geographical location distribution of vasculitis (AAV and GCA*) RCTs (n=41).
Table 1. Race, ethnicity, sex, and geographical location distribution of vasculitis (AAV and GCA*) RCTs (n=41).
.jpg) Figure 1. Geographic distribution of enrolling sites for ANCA-Associated Vasculitis (Panel A) and Giant Cell Arteritis (Panel B) RCTs worldwide.
Figure 1. Geographic distribution of enrolling sites for ANCA-Associated Vasculitis (Panel A) and Giant Cell Arteritis (Panel B) RCTs worldwide.
.jpg) Figure 2. Geographic distribution of enrolling sites for ANCA-Associated Vasculitis (Panel A) and Giant Cell Arteritis (Panel B) RCTs in US per state.
Figure 2. Geographic distribution of enrolling sites for ANCA-Associated Vasculitis (Panel A) and Giant Cell Arteritis (Panel B) RCTs in US per state.
To cite this abstract in AMA style:
Carpio Tumba M, Mohamadi A, Louden D, Pimentel-Quiroz V, Putman M, Saygin D, Lomanto Silva R, Sattui S. Current State of Racial, Ethnic, Sex, and Geographical Diversity in ANCA-associated vasculitis and Giant Cell Arteritis Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/current-state-of-racial-ethnic-sex-and-geographical-diversity-in-anca-associated-vasculitis-and-giant-cell-arteritis-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/current-state-of-racial-ethnic-sex-and-geographical-diversity-in-anca-associated-vasculitis-and-giant-cell-arteritis-trials/
