Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus Therapeutics (LT), the clinical affiliate of the Lupus Research Alliance, oversees the premier North American Lupus Clinical Investigators Network (LuCIN). As cell therapy emerges as a potentially transformative, but operationally demanding modality in lupus trials, understanding site readiness and barriers is essential. To address the anticipated expansion of cell therapy trial activity, an annual network-wide survey was conducted to assess the capability and requirements necessary for sites to engage in cell therapy clinical studies.
Methods: Using an internet-based platform, an annual survey was administered between Dec 17, 2024, and Feb13, 2025; a total of 60 LuCIN sites submitted multiple team responses. The survey asked about institutional infrastructure, interdepartmental collaboration, readiness for various cell therapy modalities, and educational/training priorities.
Results: Of the 124 respondents, enthusiasm for cell therapy was high: 60% expressed a preference to participate in Phase 2 trials while 35% in Phase 1 cell therapy trials. Chimeric antigen receptor T-cell (CAR-T) was the preferred modality (75%) followed by allogenic CAR-Natural Killer cell (44%) or other allogeneic approaches (40%) Table 1. Most sites (≥74%) reported the presence of core inpatient infrastructure (e.g., apheresis, drug storage, certified infusion providers, SOPs, toxicity monitoring), 62% had outpatient infusion capabilities, highlighting significant network readiness and evolution to adopt cell therapy for further study in lupus. Interdepartmental collaboration was active, as 66% reported partnerships with oncology/hematology and 59% seeking training to enable further collaboration. The most common operational challenges were logistical hurdles (74%), competing clinical studies (58%), and limited shared staffing capacity (39%). Meanwhile, 48% of respondents noted there are “too many” cell therapy opportunities. Investment in cell therapy capabilities, while increasing, was uneven where 59% reported enhanced multidisciplinary collaboration, 31% on additional staff or expertise and 20% reported new infrastructure, but 31% reported no new investments. Educational gaps were present as 76% requested new patient education materials, including CAR-T, with a priority on safety monitoring (66%), recruitment and patient education (64%), clinical protocol design (61%), among others (Table 2). Respondents identified target audiences of research staff (64%), patients (62%), and providers (56%) for cell therapy education opportunities.
Conclusion: Understanding readiness for cell therapy in lupus is critical to build a network of capable cell-therapy trialists for lupus and beyond. Using this knowledge, strategic investment in operational planning, capacity building and tailored education efforts for staff, patients, and providers will provide the research community support for the safe, effective and scalable integration of cell therapy in lupus trials. Careful attention to resource allocation, multidisciplinary partnerships and trial prioritization will be essential to avoid saturation and sustain momentum across LuCIN sites.
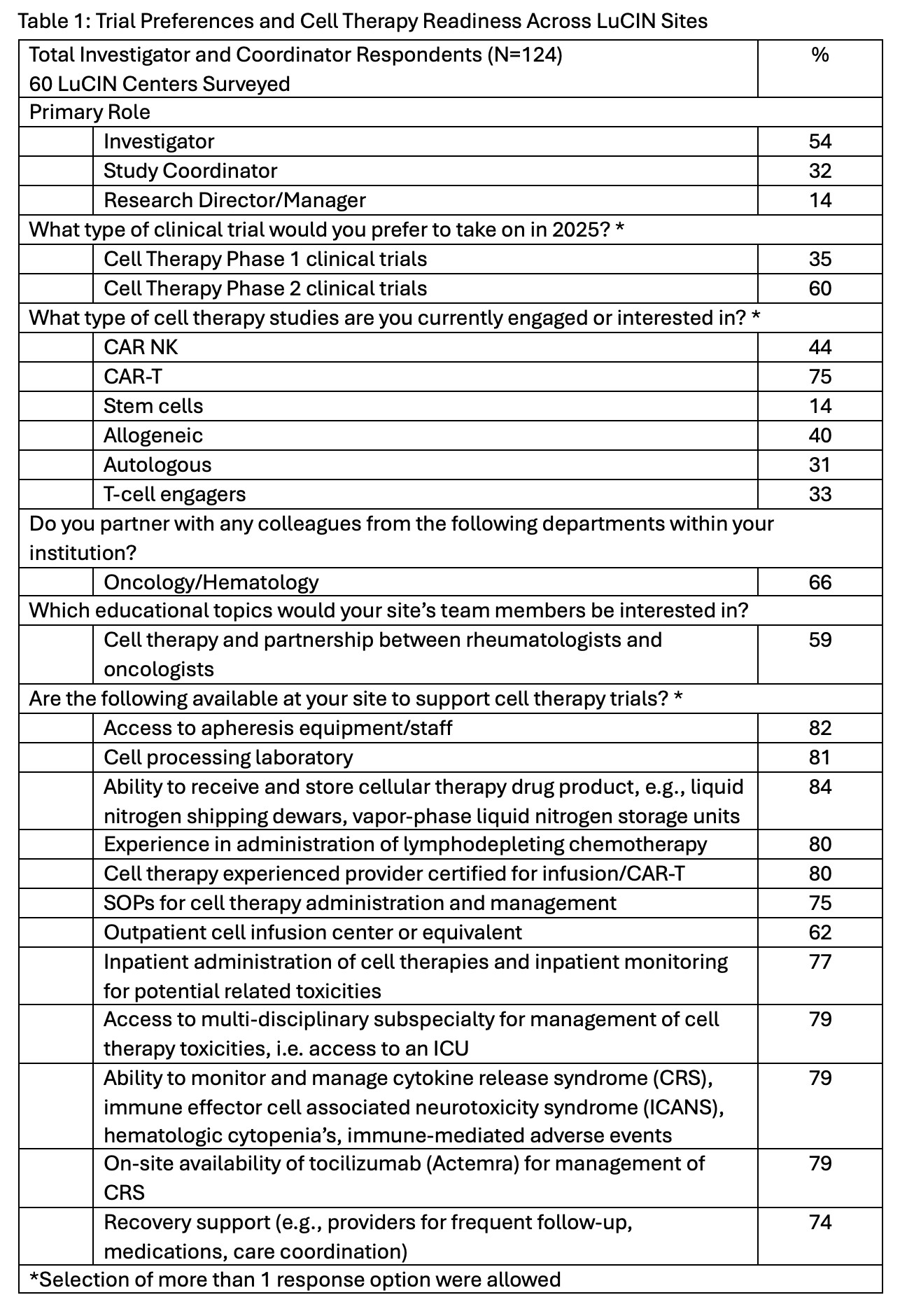 Table 1: Trial Preferences and Cell Therapy Readiness Across LuCIN Sites
Table 1: Trial Preferences and Cell Therapy Readiness Across LuCIN Sites
.jpg) Table 2: Barriers, Investments, and Educational Needs for Cell Therapy Trials
Table 2: Barriers, Investments, and Educational Needs for Cell Therapy Trials
To cite this abstract in AMA style:
Jackson B, Sheikh S, Caricchio R, Irons T, Dall'Era M, Saxena A, Kim A, Rubio J, Bernatsky S, Goddard D, Koumpouras F, Williams A, Merrell M, Meriwether J, Bell S. Evolution and Readiness: Preparing for Cell Therapy in Lupus Trials, A LuCIN Network Evaluation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evolution-and-readiness-preparing-for-cell-therapy-in-lupus-trials-a-lucin-network-evaluation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evolution-and-readiness-preparing-for-cell-therapy-in-lupus-trials-a-lucin-network-evaluation/
