Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The Phase III REGENCY study (NCT04221477) demonstrated superiority of obinutuzumab (OBI) over placebo (PBO) in achieving complete renal response (CRR) at Week 76 when added to standard therapy (ST) of mycophenolate mofetil (MMF) plus glucocorticoids in patients with active lupus nephritis (LN). Improvement or stabilization of kidney function and improvement of proteinuria are commonly included in the definitions of complete response in recent LN clinical studies, but there is no standardized definition of the CRR endpoint.In these post hoc analyses, we evaluated i) the effect of OBI+ST vs PBO+ST across different renal response definitions used in recent studies, ii) the efficacy of OBI+ST vs PBO+ST on each component included in the REGENCY CRR definition, and iii) the oral prednisone intake over time between the two treatment groups.
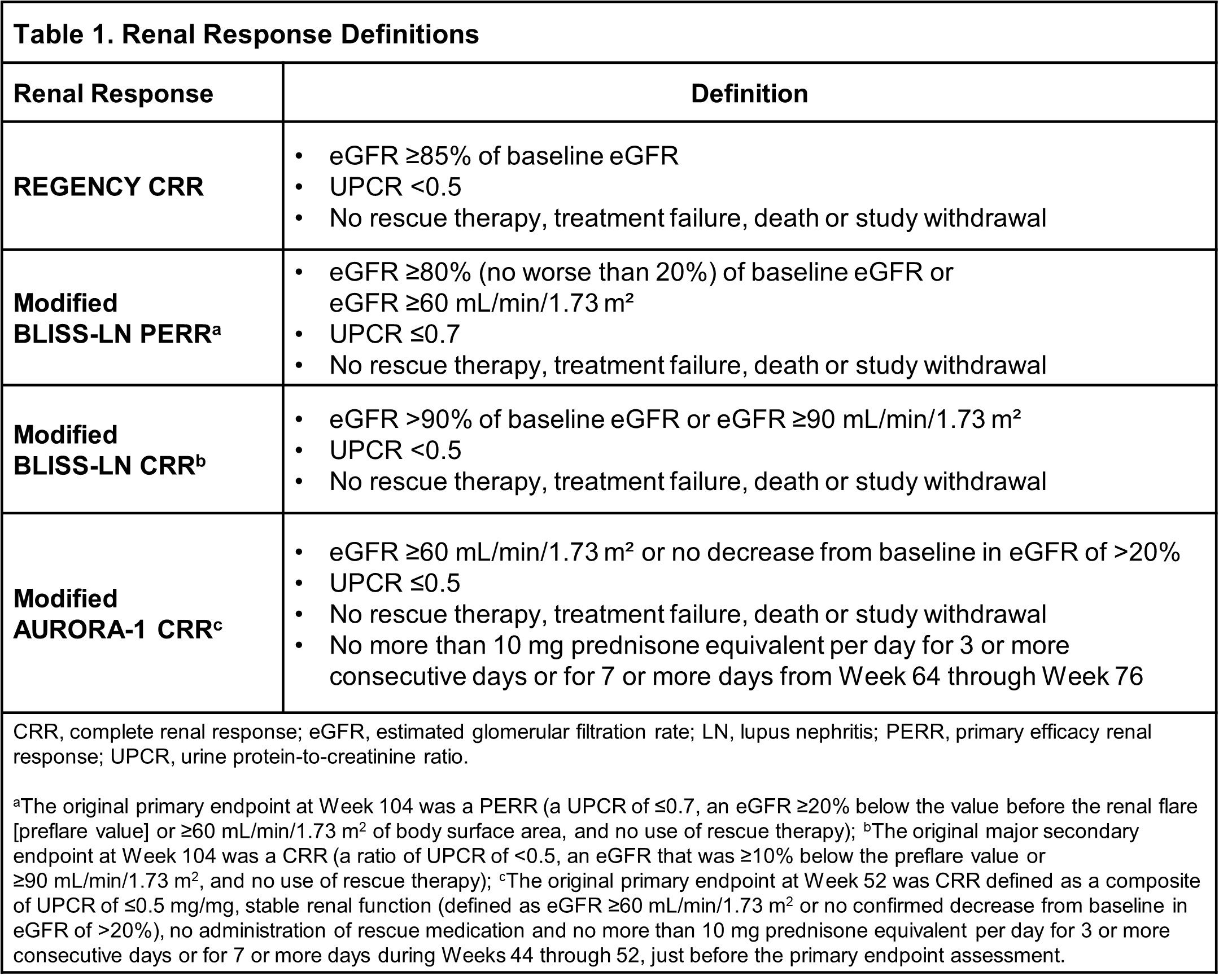
Methods: REGENCY, BLISS-LN and AURORA-1 renal response endpoint definitions were used for analysis of the REGENCY dataset at Week 76. Endpoint measure definitions are in Table 1. The Cochran–Mantel–Haenszel test statistic stratified by the IxRS stratification factors, region and race, was used for the two treatment comparisons. All patients met ACR criteria for SLE and had biopsy-proven active LN. As part of ST, all patients received oral prednisone (or equivalent) and followed a standardized tapering schedule to achieve a target dose of 5 mg/day (or equivalent) by study Week 24.
Results: In the OBI+ST (n=135) and PBO+ST groups (n=136), respectively, 46.4% and 33.1% of patients achieved CRR at Week 76 (adjusted difference 13.4%, 95% CI, 2.0 to 24.8%; P=0.0232). The proportions of patients at Week 76 achieving i) a modified BLISS-LN primary efficacy renal response were 51.8% and 39.7% (adjusted difference: 12.1%, 95% CI, 0.5 to 23.8%; P=0.0432); ii) a modified BLISS-LN CRR were 48.7% and 33.1% (adjusted difference: 15.7%, 95% CI, 4.3 to 27.2%; P=0.0084); iii) a modified AURORA-1 CRR were 48.7% and 33.8% (adjusted difference: 15.0%, 95% CI, 3.6 to 26.5%; P=0.0117) (Figure 1).In REGENCY, the proportions of patients achieving the individual components of CRR at Week 76 were numerically higher in the OBI arm compared with the PBO arm for all three components (UPCR < 0.5 g/g: 47.4% vs 36.0%; eGFR ≥85% of baseline: 83.7% vs 75.7%; no occurrence of intercurrent events: 88.9% vs 75.0% for OBI+ST and PBO+ST, respectively). In both arms, the main reasons for not attaining CRR were a UPCR ≥0.5 or an eGFR < 85% of baseline (54.8% OBI+ST vs 65.4% PBO+ST).The mean daily prednisone intake was consistently lower in patients in the OBI arm compared with the PBO arm from Week 24-76, although the 95% CI overlapped at some intermediate visits (Figure 2). The proportions of patients achieving a daily prednisone (or equivalent) dose of ≤5 mg/day were consistently higher in the OBI vs PBO arm from Week 36. This reached a 10-percentage point absolute difference from Week 64-76 (78.5% vs 68.4% for OBI+ST vs PBO+ST, respectively; adjusted difference (95% CI): 10.1% (−0.5 to 20.4), P=0.0589).
Conclusion: In a post hoc analysis of the REGENCY trial outcomes, OBI showed consistent benefit across various patient subgroups, utilizing multiple alternative definitions of CRR, and also exhibited steroid-sparing properties.
To cite this abstract in AMA style:
Rovin B, Garg J, Furie R, Jones R, Saxena A, Esposito P, Martins E, Petry C, Frey N, Yoo B, Hassan I, Schindler T, Omachi T, Pendergraft W, Santiago M, Aroca Martínez G, Malvar A. Obinutuzumab Demonstrates Steroid-Sparing Effects and Consistent Benefit In Patients with Lupus Nephritis When Using Multiple Primary Endpoint Definitions: A Secondary Analysis of Phase III Trial Results [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/obinutuzumab-demonstrates-steroid-sparing-effects-and-consistent-benefit-in-patients-with-lupus-nephritis-when-using-multiple-primary-endpoint-definitions-a-secondary-analysis-of-phase-iii-trial-resu/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/obinutuzumab-demonstrates-steroid-sparing-effects-and-consistent-benefit-in-patients-with-lupus-nephritis-when-using-multiple-primary-endpoint-definitions-a-secondary-analysis-of-phase-iii-trial-resu/

.jpg)
.jpg)