Session Information
Date: Sunday, October 26, 2025
Title: (0593–0640) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: For childbearing women with SLE and other autoimmune diseases, the nearly invariant association of cardiac-NL with maternal SSA/Ro52/60kD autoantibodies supports the necessity of these reactivities in the pathogenesis. Although high titer and a history of a previously affected child further increase the risk of fetal disease, the incidence is < 20%, suggesting that another factor, be it protective or permissive, is operative. Addressing the latter, a novel antigenic target Na+/K+ ATPase subunit α1 (ATP1A1), which maintains ion balance across cell membranes, was discovered by western blot screening of fetal hearts and stem-cell derived immature cardiomyocytes, and found to be uniquely targeted only in pregnancies affected by cardiac-NL (Hamilton, ACR, 2022). To facilitate high throughput clinical assessments of this specificity, we leveraged an extensive collection of sera from high titer anti-Ro pregnancies, both affected and unaffected by cardiac-NL, to evaluate the utility of an ELISA based approach to detect anti-ATP1A1 for risk evaluation (Fig 1).
Methods: Commercially available full-length ATP1A1 was coated at a concentration of 0.2 ug/well. All sera were diluted 1:1000. The patient with the highest OD on the first plate was tested on all subsequent plates and all samples were divided by this calibrator to generate a normalized OD. Positive reactivity was defined as 2SD above the mean normalized OD obtained for 24 sera from healthy anti-Ro negative first trimester pregnancies. Sources of all samples are shown in Figure 1.
Results: Of the 46 anti-Ro positive sera tested during pregnancies affected by cardiac-NL (18 in the early second trimester prior to the identification of a fetal arrhythmia), 65% were reactive with full length ATP1A1 (Fig 2A & 2B). Comparable frequencies were observed in 39 anti-Ro positive sera tested during the second trimester of cardiac healthy fetuses, specifically 64%. Similarly, on a continuous scale, ATP1A1 levels did not distinguish cardiac-NL from healthy outcomes (Fig 2A & 2B). To address whether anti-ATP1A1 may be present only during affected pregnancies, unlike anti-Ro antibodies, we evaluated 15 pairs in which one pregnancy was healthy and another affected in the same individual. There were no significant differences between ATP1A1 levels in the affected vs unaffected pregnancy (Fig 2C). One patient became seropositive in the affected pregnancy, while one other lost reactivity in the affected pregnancy (Fig 2C). ATP1A1 levels correlated with anti-Ro52 (r=0.8, p < 0.0001) and anti-Ro60 (r=0.5, p< 0.0001) titers. Consistent with this association, reactivity with ATP1A1 was not detected in any of six anti-Ro negative SLE pregnancies.
Conclusion: These data suggest that autoantibodies against full length ATP1A1, measured by ELISA, are present in the majority of anti-Ro+ pregnancies and represent a novel reactivity. However, as tested, this reactivity does not predict the development of cardiac-NL. Binding specific epitopes in the western blot could differ from our approach. The strong correlation with anti-Ro52 implicates cross-reactivity between these targets.
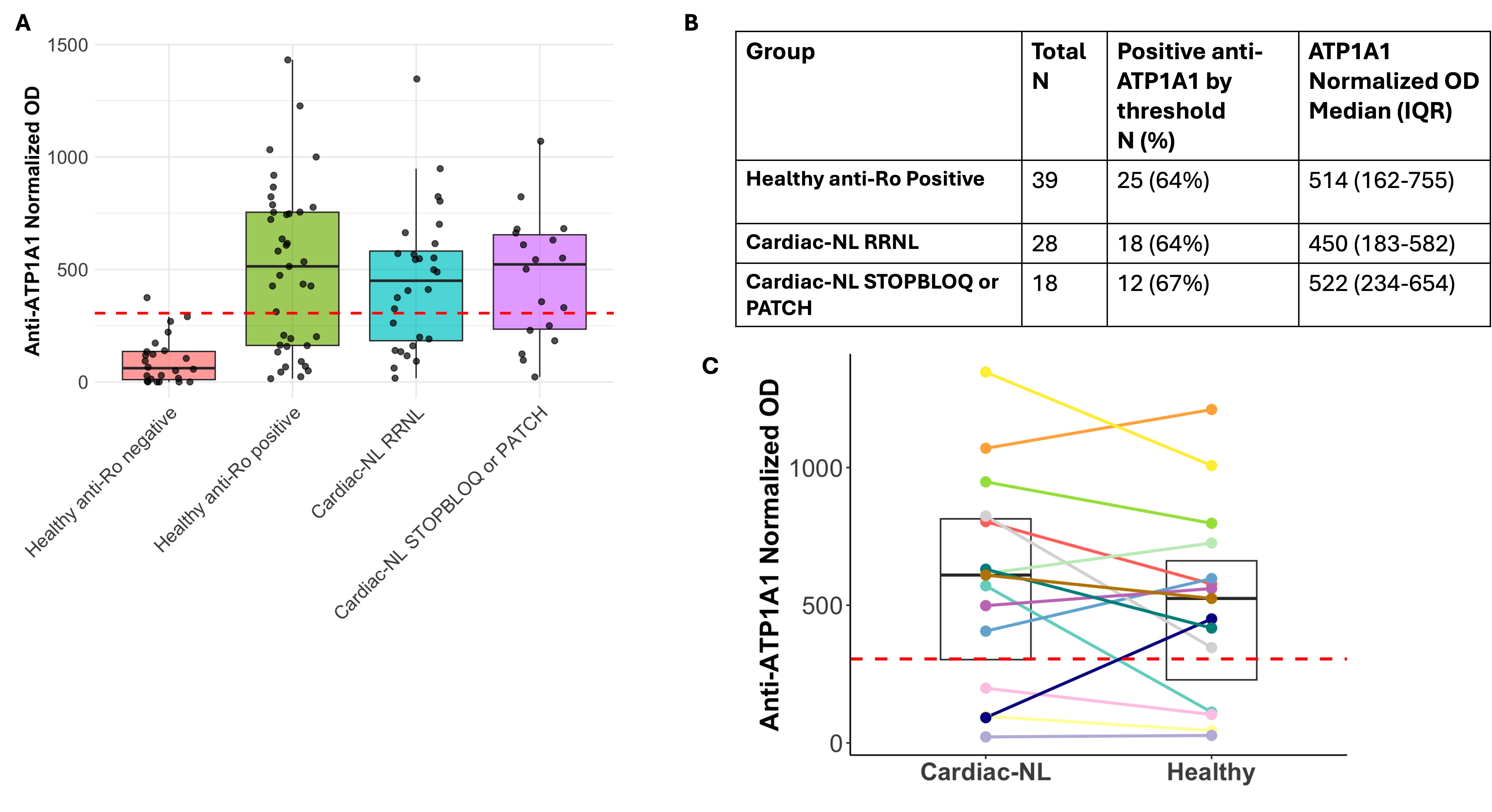 Figure 1: Flow chart describing the sources of the samples used in the study. RRNL: Research Registry for Neonatal Lupus; STOP BLOQ: Surveillance and Treatment to Prevent AV Block Likely to Occur Quickly; PATCH: Preventive Approach To Congenital Heart Block with Hydroxychloroquine
Figure 1: Flow chart describing the sources of the samples used in the study. RRNL: Research Registry for Neonatal Lupus; STOP BLOQ: Surveillance and Treatment to Prevent AV Block Likely to Occur Quickly; PATCH: Preventive Approach To Congenital Heart Block with Hydroxychloroquine
.jpg) Figure 2: (A) Boxplots showing the normalized optical density (OD) values for the healthy anti-Ro negative, healthy anti-Ro positive, Research Registry for Neonatal Lupus (RRNL) cardiac-NL, and STOP BLOQ or PATCH cardiac-NL samples. The red dashed line at 305 indicates mean + 2 standard deviations above the healthy anti-Ro negative samples. (B) Table showing the percent of patients in each group above the 305 threshold and the median and interquartile range. (C) Boxplots showing the ATP1A1 levels between paired samples collected from a patient during a cardiac-NL (RRNL, PATCH, or STOP BLOQ) and a healthy pregnancy.
Figure 2: (A) Boxplots showing the normalized optical density (OD) values for the healthy anti-Ro negative, healthy anti-Ro positive, Research Registry for Neonatal Lupus (RRNL) cardiac-NL, and STOP BLOQ or PATCH cardiac-NL samples. The red dashed line at 305 indicates mean + 2 standard deviations above the healthy anti-Ro negative samples. (B) Table showing the percent of patients in each group above the 305 threshold and the median and interquartile range. (C) Boxplots showing the ATP1A1 levels between paired samples collected from a patient during a cardiac-NL (RRNL, PATCH, or STOP BLOQ) and a healthy pregnancy.
.jpg) Figure 3: Correlation between Anti-ATP1A1 and Anti-Ro52 (A) or Anti-Ro60 (B). Pearson’s correlation coefficient and p-value are listed on the graphs.
Figure 3: Correlation between Anti-ATP1A1 and Anti-Ro52 (A) or Anti-Ro60 (B). Pearson’s correlation coefficient and p-value are listed on the graphs.
To cite this abstract in AMA style:
Sachan N, Carlucci P, Masson M, Fraser N, Izmirly P, Hernández-Blanco C, Clancy R, Cuneo B, Buyon J. Evaluation of Novel Anti-ATP1A1 Autoantibodies by ELISA to Predict Cardiac Neonatal Lupus (Cardiac-NL) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-novel-anti-atp1a1-autoantibodies-by-elisa-to-predict-cardiac-neonatal-lupus-cardiac-nl/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-novel-anti-atp1a1-autoantibodies-by-elisa-to-predict-cardiac-neonatal-lupus-cardiac-nl/
