Session Information
Date: Sunday, October 26, 2025
Title: (0593–0640) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Treat-to-target (T2T) states in SLE, such as the Lupus Low Disease Activity State (LLDAS) and remission (REM) as defined by the Definitions of Remission in SLE (DORIS), are now reflected in treatment guidelines. However, while sustained attainment of these states is reported in response to CD19 CAR-T treatment, limited long-term data exist quantifying the frequency of sustained attainment of these states on standard of care.
Methods: We examined the cumulative and sustained (continuous) attainment of LLDAS, REM, and steroid-free remission (REM-0) utilising the Asia Pacific Lupus Collaboration (APLC) patient cohort. Data were collected from 25 sites across 13 countries between 1st January 2013 and 31st December 2023 using standardised templates. LLDAS was defined as Golder et al., 2019 (1) (SLEDAI≤4, no new activity, PGA ≤1, prednisolone (PNL) ≤7.5 mg/d, antimalarials (AM) and immunosuppressants (IS) allowed). REM was defined as Vollenhoven et al, 2021 (2) (clinical SLEDAI=0, PGA < 0.5, PNL≤5 mg/d, AM/ IS allowed), while REM-0 was defined as clinical SLEDAI=0, PGA < 0.5, PNL=0 mg/d, AM/ IS allowed. We examined these outcomes in all patients, and in patients who at enrolment were not at target (i.e. not in LLDAS).
Results: A total of 68,083 visits from 5,053 patients with ≥2 visits were evaluated. About 82% of patients attained LLDAS; ~70% attained REM, and 31% attained REM-0 at least once during the observation period. Accordingly, LLDAS was achieved in 48% of visits (n= 31,706); REM in 37% of visits (n= 31,706), and REM-0 in 13.5% of visits (9,189) (Figure 1). Cumulative and sustained attainment of these T2T states were low, especially sustained REM-0 (~14%) (Table 1). Although the proportion of patients attaining sustained REM-0 gradually increased over time, the majority of patients never achieved this state (Figure 2). Overall, patients who attained sustained REM0 were older, had a longer study duration, and had used more AM (88% vs. 84% of sustained REM0-never patients, p=0.015).We further examined the attainment of these T2T states in 2,517 patients who were not at target (i.e. not in LLDAS) at enrolment (51% of the cohort). These patients were younger and had shorter SLE disease duration than those in LLDAS at enrolment. Fewer of these patients attained T2T states; only 18% achieved REM-0 at least once, and < 6% achieved 12 months sustained REM-0 (Table 1).
Conclusion: Sustained attainment of steroid-free remission was infrequent in SLE patients receiving standard of care.References1. Golder V, Kandane-Rathnayake R, Huq M, Nim H, Louthrenoo W, Luo SF, et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. The Lancet Rheumatology. 2019;1(2):e95–e102.2. van Vollenhoven RF, Bertsias G, Doria A, Isenberg D, Morand E, Petri MA, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med. 2021;8(1).
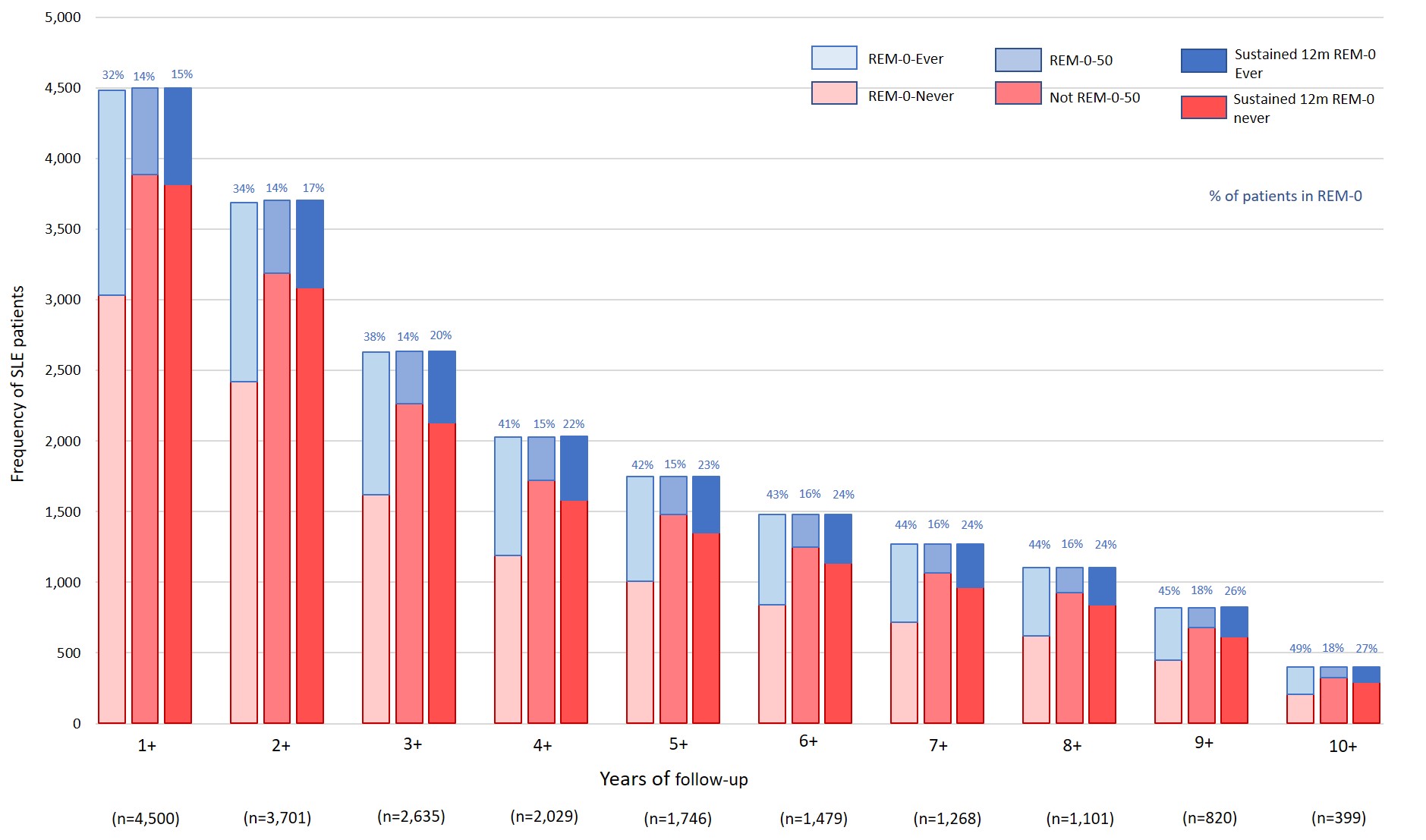 Table 1 – Patient characteristics
Table 1 – Patient characteristics
.jpg) Figure 1: Patient visits fulfilling REM-0
Figure 1: Patient visits fulfilling REM-0
.jpg) Figure 2 – Attainment of steroid-free remission (REM-0) overtime
Figure 2 – Attainment of steroid-free remission (REM-0) overtime
To cite this abstract in AMA style:
Kandane-Rathnayake R, Chen Y, Hoi A, Golder V, Louthrenoo W, Cho J, Lateef A, Hamijoyo L, Luo S, Wu Y, Chan C, Zamora L, Katsumata Y, Sockalingam S, Li Z, Yao H, Basnayake B, Jia Poh Y, Hao Y, Zhang Z, Chan M, Kikuchi J, Kaneko Y, Takeuchi T, Oon S, Ng K, Bae S, Tee C, Tee M, Tugnet N, O’Neill S, Hassett G, Goldblatt F, Ohkubo N, Miyazaki Y, Sapsford M, Tanaka Y, Navarra S, Lau C, Nikpour M, Morand E. Sustained Remission In SLE Is Infrequent On Standard Of Care: A Decade Of Insights From The Asia Pacific Lupus Collaboration [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sustained-remission-in-sle-is-infrequent-on-standard-of-care-a-decade-of-insights-from-the-asia-pacific-lupus-collaboration/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-remission-in-sle-is-infrequent-on-standard-of-care-a-decade-of-insights-from-the-asia-pacific-lupus-collaboration/
