Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Sonelokimab (SLK), a novel Nanobody that binds to both IL-17A and IL-17F with similarly high affinity, is designed to target difficult-to-reach sites of inflammation. The Phase 2 ARGO study demonstrated robust joint and skin responses with SLK in PsA. We report Week (W) 24 data for patients with prior biologic exposure in the ARGO trial and describe the design of the Phase 3 IZAR-2 trial that will further evaluate SLK in patients with active PsA after prior biologic TNFi therapy.
Methods: ARGO (NCT05640245) was a 24-week, global, randomized, placebo (PBO)-controlled double-blind trial of adults with active PsA. Three SLK doses were assessed: 60mg with no induction (NI; Q4W), SLK 60mg with induction (WI), and SLK 120mg WI (induction doses at W0, 2, 4, and 6; Q4W from W8). Patients with >2 prior biologics or primary failure of TNFi or IL-17i were excluded. In this exploratory analysis, W24 outcomes in patients with prior bDMARD exposure treated with SLK 120mg or 60mg WI (IZAR-2 doses) were assessed.
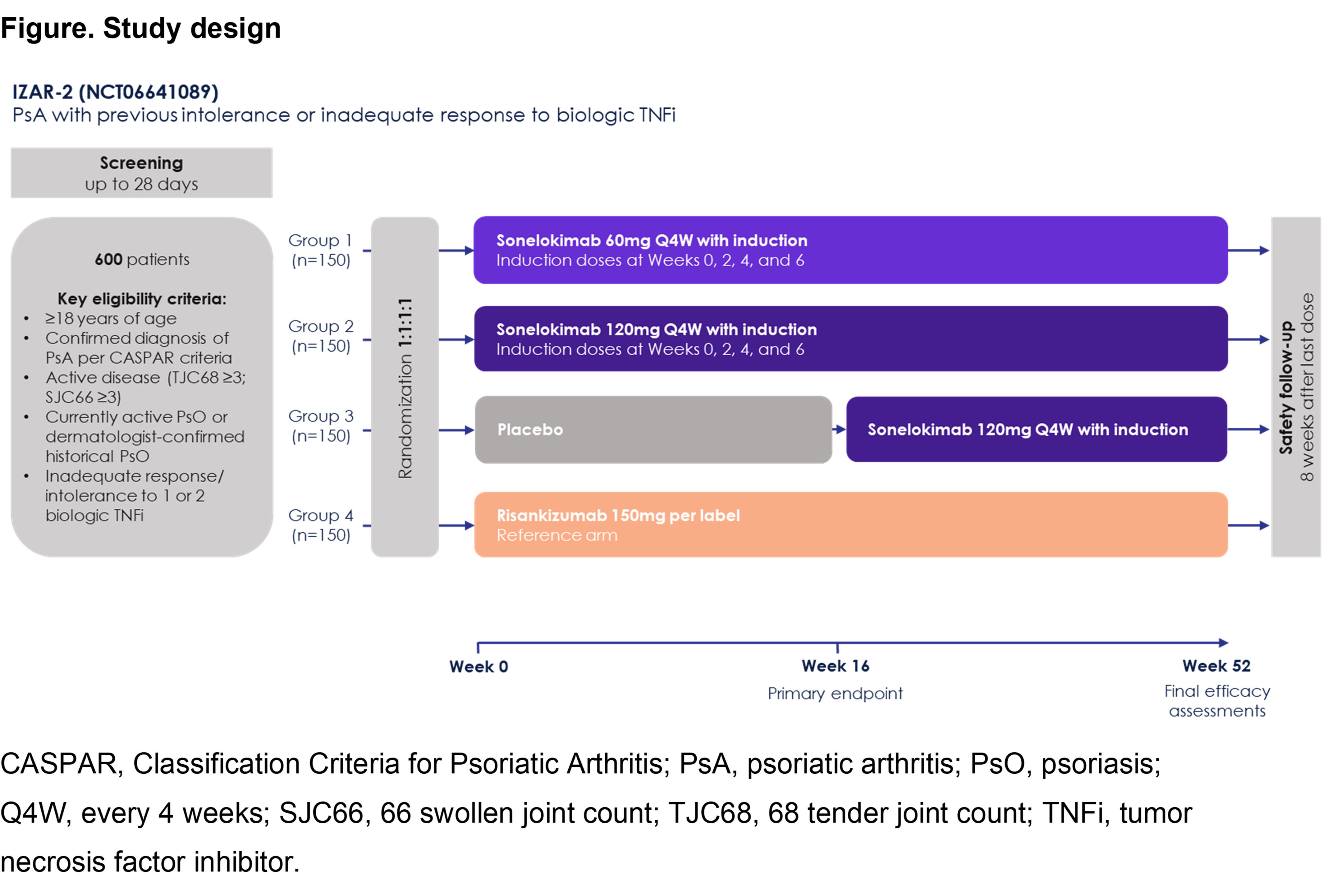
Results: 207 patients were enrolled. Of the 84 patients treated with SLK 120mg (n=43) or 60mg WI (n=41), 15 (17.9%) had exposure to ≥1 bDMARD (TNFi n=10, IL-23i n=7, IL-17i n=5). Among these patients, the primary endpoint of ACR50 was achieved by 53% of patients with exposure to ≥1 bDMARD and 61% of patients with no prior bDMARD exposure. Similar results were seen for minimal disease activity (MDA), which was achieved by 53% of patients with prior bDMARD exposure and 57% of patients with no prior bDMARD exposure who received SLK 120mg or 60mg WI. Of the 5 patients receiving SLK 120mg or 60mg WI with PsO at baseline (≥3% BSA) and prior bDMARD exposure, 4 (80%) achieved PASI 90 (75% for those with no prior bDMARD exposure). SLK was well tolerated, with a safety profile consistent with IL-17 inhibition. To further assess outcomes in this patient population, we designed a 52-week, global, randomized, double-blind, PBO-controlled, Phase 3 trial (IZAR-2; NCT06641089; Figure). An estimated 600 participants will be randomized 1:1:1:1 to SLK 60mg WI, SLK 120mg WI, PBO (SLK 120mg WI from W16), or risankizumab (RZB). Given the results from ARGO, this sample size should provide sufficient power to demonstrate a statistically significant difference vs. PBO on the primary outcome of ACR50 at W16. Eligible participants will have PsA fulfilling CASPAR criteria, active disease (TJC68 ≥3 and SJC66 ≥3), currently active or historical PsO, and inadequate response or intolerance to 1 or 2 biologic TNFi. Key secondary outcomes (all vs. PBO at W16) include ACR20, PASI 90, MDA, and HAQ-DI. RZB, an approved IL-23 p19i PsA therapy commonly used after prior inadequate response to TNFi, will be the active reference arm. A secondary endpoint of ACR50 at W16 with SLK vs. RZB will provide insights into treatment choices with IL-17A/F and IL-23i after TNFi failure.
Conclusion: In patients with active PsA and prior exposure to >1 bDMARD in the Phase 2 ARGO trial, SLK 120mg and 60mg WI showed efficacy in controlling signs and symptoms of PsA and achieving MDA response. Interpretation is limited by patient numbers; hence, a larger Phase 3 trial specifically in patients with prior inadequate response or intolerance to biologic TNFi (IZAR-2) is ongoing and actively recruiting at sites across the USA.
To cite this abstract in AMA style:
Deodhar A, Gossec L, Mease P, Baraliakos X, Eder L, Kivitz A, Marzo-Ortega H, Behrens F, Orbai A, Schett G, Kavanaugh A, McGonagle D, Ritchlin C, Brennan N, Porter-Brown B, Cullen E, Thomas M, Albulescu M, Godwood A, Reich K, Coates L. Sonelokimab in Biologic-Experienced Patients With Active Psoriatic Arthritis: Results From a Phase 2 Trial (ARGO) and Study Design of a Phase 3 Trial (IZAR-2) in Patients With Inadequate Response or Intolerance to Biologic TNFi, Including a Risankizumab Reference Arm [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/sonelokimab-in-biologic-experienced-patients-with-active-psoriatic-arthritis-results-from-a-phase-2-trial-argo-and-study-design-of-a-phase-3-trial-izar-2-in-patients-with-inadequate-response-or-i/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sonelokimab-in-biologic-experienced-patients-with-active-psoriatic-arthritis-results-from-a-phase-2-trial-argo-and-study-design-of-a-phase-3-trial-izar-2-in-patients-with-inadequate-response-or-i/