Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Since most approved biologics are administered subcutaneously, there is a need for alternate routes of delivery to treat patients with SpA. Secukinumab is an IL-17A inhibitor that was additionally approved for IV administration for SpA. This study described the demographic, clinical and treatment characteristics of patients with SpA who initiated IV secukinumab in real-world clinical settings.
Methods: This retrospective cohort study using HealthVerity claims database included patients who initiated IV secukinumab between Oct 6, 2023 and Oct 31, 2024 (index date = first record), with ≥1 SpA diagnosis code, including psoriatic arthritis (PsA) and axial SpA (axSpA) in 12 months prior to or up to 14 days after index date. Patients had ≥12 months of continuous enrollment (CE) prior to the index date (baseline period) and ≥3 months post-index CE. Patient demographic, clinical, and treatment characteristics were described at the index date, within 12 months prior to index date, or beyond 12 months (baseline period), as indicated. Traditional therapies included corticosteroids, NSAIDS, or conventional synthetic (cs)DMARDs. Advanced therapies included biologic (b)DMARDs, targeted synthetic (ts)DMARDs, or both during the baseline period. Time from the start and end of therapy prior to the index date is reported.
Results: Overall, 255 patients with SpA who initiated IV secukinumab met the eligibility criteria and were included. Most patients were diagnosed with PsA (65.5%), 25.5% of patients were diagnosed with axSpA, and 9.0% of patients had both PsA and axSpA. The mean (SD) age at index date was 55.5 (13.1.) years, and 73.3% were female (Table 1). During the 12 months prior to index, the mean (SD) Deyo-Charlson Comorbidity Index score (DCCI) was 2.8 (2.6), and 43.9% of patients had a score of >3.0. Common comorbidities included osteoarthritis/osteopenia (64.7%), neurological disorder (56.1%), plaque psoriasis (52.2%), and obesity (48.6%). The median (interquartile range [IQR]) time from first observed SpA diagnosis to index was 34.6 (14.7-66.7) months. During the 12 months prior to index, 73.7% of all patients used traditional therapies, which included corticosteroids (65.1%), NSAIDs (36.9%) and csDMARDs (30.2%, Table 2). The median (IQR) time from first supply of traditional therapy to index was 64.3 (31.0-89.9) months and from last supply was 1.9 (0.5-8.7) months. During the 12 months prior to index, advanced therapies were used by 37.3% of all patients, which included bDMARDs (33.3%), tsDMARDS (9.0%), or a combination of both (5.1%). The median (IQR) time from first supply of advanced therapy to initiation of IV secukinumab was 34.2 (17.2-62.3) months and from last supply was 5.4 (2.2-14.1) months.
Conclusion: In this population of patients with SpA who initiated IV secukinumab, patients were generally older with longstanding disease and high prevalence of comorbidities (e.g., osteoarthritis/osteopenia, neurological disorder, plaque psoriasis, or obesity), which underscores the complexity of SpA disease burden in a diverse group of patients with varied characteristics and health conditions. IV secukinumab was initiated soon after previous treatments, possibly in response to disease progression.
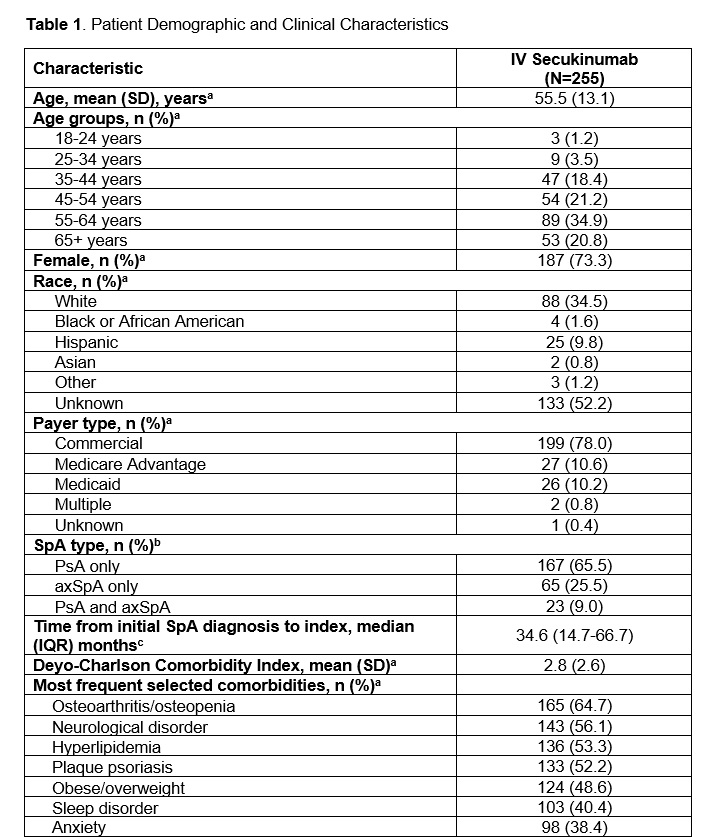 axSpA, axial spondyloarthritis; IQR, interquartile range; IV, intravenous; PsA, psoriatic arthritis; SpA, spondyloarthritis.
axSpA, axial spondyloarthritis; IQR, interquartile range; IV, intravenous; PsA, psoriatic arthritis; SpA, spondyloarthritis.
a Measured at index date or < 12 months prior to index.
b Measured at index date, < 12 months prior to index, or up to 14 days after index date.
c Measured < 12 months or ≥12 months prior to index.
.jpg) bDMARDs, biologic disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; IQR, interquartile range; IV, intravenous; NSAIDs, non-steroidal anti-inflammatory drugs; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs.
bDMARDs, biologic disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; IQR, interquartile range; IV, intravenous; NSAIDs, non-steroidal anti-inflammatory drugs; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs.
a Measured < 12 months or ≥12 months prior to index.
To cite this abstract in AMA style:
Kivitz A, Grinnell-Merrick L, Nguyen T, Faucher A, Taiji R, Vekeman F, Singhal A. Patient Characteristics and Treatment Patterns of Traditional and Advanced Therapies Prior to First Secukinumab Intravenous (IV) Administration in Patients with Spondyloarthritis (SpA) in US Real-World Clinical Settings [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/patient-characteristics-and-treatment-patterns-of-traditional-and-advanced-therapies-prior-to-first-secukinumab-intravenous-iv-administration-in-patients-with-spondyloarthritis-spa-in-us-real-worl/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-characteristics-and-treatment-patterns-of-traditional-and-advanced-therapies-prior-to-first-secukinumab-intravenous-iv-administration-in-patients-with-spondyloarthritis-spa-in-us-real-worl/
