Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical II (0879–0884)
Session Type: Abstract Session
Session Time: 10:30AM-10:45AM
Background/Purpose: Circulating monocytes with pro-inflammatory and pro-fibrotic phenotype have been implicated in SSc-ILD pathogenesis, but their potential trajectory to mature cell lineage macrophages driving organ-specific damage is not well defined.Evidence suggests that osteopontin expressing macrophages likely contribute to fibrotic mechanisms in SSc-ILD. We sought to characterize specific myeloid subpopulations associated with SSc-ILD progression and establish potential precursor relationships with lung macrophages.
Methods: Single-cell RNA-seq (scRNA-seq) was performed on peripheral blood obtained from 60 SSc patients meeting 2013 ACR/EULAR criteria. Unsupervised clustering identified two subpopulations of monocytes with type I interferon signatures. Differential expression and subtype correlation analyses against %change in forced vital capacity (FVC, liter) per year from baseline were conducted in subjects with SSc-ILD (n=40). Transcription factor (TF) analysis was conducted for progressive ( >10% decrease in FVC) and non-progressive SSc-ILD patients. Reprocessed publicly available scRNA-seq data from SSc-ILD lungs were used to perform trajectory analysis.
Results: Clustering and annotation of myeloid cells identified 11 myeloid cell types and two subclusters (CD74+ and CD74-) of an “ISG15-hi” monocyte population expressing high levels of interferon (IFN)-inducible markers (Fig.1A). The CD74+ subpopulation showed further enrichment for genes involved in “Response to type I IFN signaling” (FDR = 6.82 x 10-4) whilst the CD74- subpopulation upregulated genes involved in “Leukocyte cell-to-cell adhesion” (FDR = 3.61 x 10-3) (Fig.1B,D). Correlation analysis of cell type proportions against FVC %-change for SSc-ILD patients (Fig.1C) revealed a Pearson correlation of 0.60 (p=1.9×10-4) in the CD74+ “ISG15-hi” monocyte population. In the CD74+ subpopulation, progressive SSc-ILD patients upregulated genes involved in “Myeloid differentiation” (FDR = 2.90 x 10-2) (Fig.2A) and further showed increased activity for TFs previously reported as specific regulators in osteopontin expressing lung macrophages (Mac-SPP1-lung) from SSc-ILD patients including ATF5 and MAFF (Fig.2B). A reanalysis of SSc-ILD lung scRNA-seq data identified a population of “ISG15-hi” classical monocytes CMos-ISG15-lung and the previously reported Mac-SPP1-lung (Fig.3A). Trajectory analysis predicted a differentiation trajectory from CMos-ISG15-lung to Mac-SPP1-lung (Fig. 3B).
Conclusion: We identified a novel CD74⁺ subcluster within ISG15-high circulating monocytes, marked by strong type I IFN-inducible gene expression and significantly negatively associated with progressive SSc-ILD. This population may serve as a precursor to pro-fibrotic, osteopontin-expressing lung macrophages, suggesting a pathogenic role in SSc-ILD.
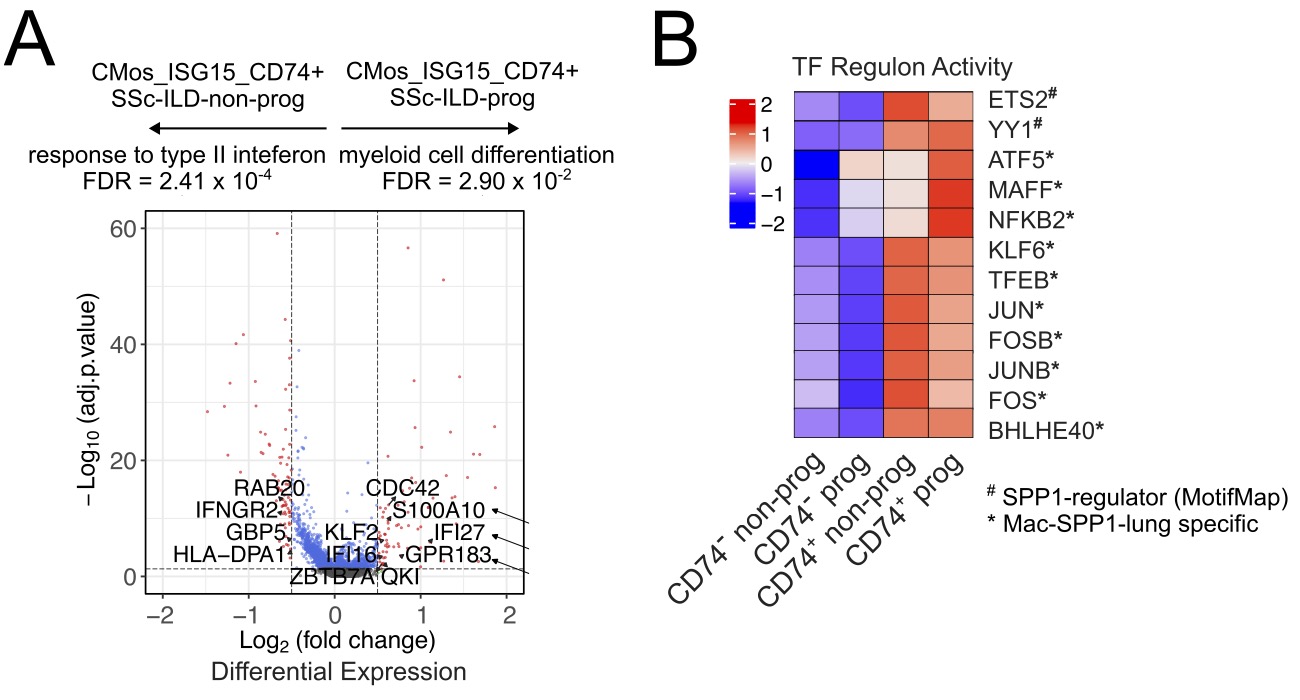 Fig. 1. A. UMAP of myeloid cells showing subclustering of monocyte cluster with type I IFN signature. B. Volcano plot of subcluster markers. C. Correlation of cluster frequencies with change in %fvc from baseline for SSc-ILD patients. D. Pathways for classical monocyte ISG15 subclusters.
Fig. 1. A. UMAP of myeloid cells showing subclustering of monocyte cluster with type I IFN signature. B. Volcano plot of subcluster markers. C. Correlation of cluster frequencies with change in %fvc from baseline for SSc-ILD patients. D. Pathways for classical monocyte ISG15 subclusters.
.jpg) Fig. 2. A. Volcano plot for differential expression of progressive vs non-progressive SSc-ILD in CD74+ subpopulation. B. SCENIC TF analysis of subclusters split by disease progression.
Fig. 2. A. Volcano plot for differential expression of progressive vs non-progressive SSc-ILD in CD74+ subpopulation. B. SCENIC TF analysis of subclusters split by disease progression.
.jpg) Fig. 3. A. DotPlot for markers of lung monocytes and osteopontin expressing macrophages. B. UMAP of clusters and trajectory analysis. Ref 1: Ann Rheum Dis. 2019 Aug 12;78(10):1379_1387
Fig. 3. A. DotPlot for markers of lung monocytes and osteopontin expressing macrophages. B. UMAP of clusters and trajectory analysis. Ref 1: Ann Rheum Dis. 2019 Aug 12;78(10):1379_1387
To cite this abstract in AMA style:
Lin S, Taylor K, Yang S, Chen Q, Alexander S, Cao Y, Sasaki T, Rao D, Bottini N, Boin F, Ainsworth R. Circulating Monocytes with High Interferon Signature as Precursors to Osteopontin Expressing Lung Macrophages in Patients with Systemic Sclerosis and Progressive Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/circulating-monocytes-with-high-interferon-signature-as-precursors-to-osteopontin-expressing-lung-macrophages-in-patients-with-systemic-sclerosis-and-progressive-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/circulating-monocytes-with-high-interferon-signature-as-precursors-to-osteopontin-expressing-lung-macrophages-in-patients-with-systemic-sclerosis-and-progressive-interstitial-lung-disease/
