Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Therapies (0873–0878)
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: QX002N is a novel high-affinity monoclonal antibody (mAb) that selectively targets IL-17A.In the phase II clinical study, QX002N was well tolerated and rapidly reduced symptoms and signs of active radiographic axial spondyloarthritis (r-axSpA) compared with placebo.We conducted the phase Ⅲ pivotal study to confirm the efficacy and safety of QX002N in r-axSpA patients. A pre-planned primary analysis was performed after all patients completed week 16. Here, we report the primary analysis results of the study. Results of exploratory radiographic endpoints at 16 weeks are also presented, which are measured by the Spondyloarthritis Research Consortium of Canada (SPARCC) Magnetic Resonance Imaging (MRI) index, an objective and quantifiable variable to assess the post-treatment inflammation changes of the Sacroiliac Joints (SIJ) and the spine.
Methods: This is a multicenter, phase III, randomized, double-blind, placebo-controlled, parallel-group study. Adults with active r-axSpA were randomized 1:1 to receive 160 mg subcutaneous QX002N every 4 weeks (Q4W) or placebo. At week 16, all patients received 160mg QX002N Q4W through week 52. The primary endpoint was the proportion of participants reaching the standard of the Assessment in SpondylArthritis International Society (ASAS) 40 at week 16. The key secondary endpoint was the proportion of participants achieving ASAS20 at week 16. The exploratory endpoints included changes from baseline in SIJ and spinal SPARCC Scores at Week 16 in patients with evaluable MRI at baseline and week 16(MRI completers).
Results: A total of 641 r-axSpA patients were randomized to receive 160mg QX002N Q4W (n=322) and placebo (n = 319). Baseline characteristics were similar between treatment groups (Table 1).Statistically significant improvements in disease activity, function, quality of life, spinal and SIJ MRI–evident inflammation were observed after 16 weeks of QX002N treatment versus placebo (Figure 1). At week 16, significantly higher proportions of QX002N patients (40.4%; P<0.0001) had achieved an ASAS40 response versus the placebo group (18.9%) (Figure 1A). Similarly, ASAS20 response rate (65.2% vs 41.3%) and other secondary endpoints all favoured QX002N versus placebo at week 16 (Figure 1B-D,G). For the MRI sub-study, 30.9% (198/641) and 30.6% (196/641) of the patients had SPARCC SIJ and spine assessments respectively. Rapid reduction from baseline to Week 16 in mean SPARCC SIJ score (QX002N:–6.2 , PBO: –2.3 ; P=0.0262; Figure 1E) and mean SPARCC spine score (QX002N: –8.1, PBO: –1.4; P=0.0005; Figure 1F) were observed.The overall incidence of Treatment-emergent adverse events (75.8% vs. 71.2%) were comparable in patients receiving QX002N and placebo. Serious adverse events were reported in 2.2% and 0.9% of patients in the QX002N and placebo groups, respectively. The majority of TEAEs were categorized as CTCAE (Version 5.0) Grade 1 or 2 (Table 2).
Conclusion: QX002N provided significant improvements in the symptoms and signs, as well as marked reduction in inflammation of the spinal and SIJ as measured by MRI in r-axSpA patients, when compared to placebo at week 16. The safety of QX002N was tolerable.
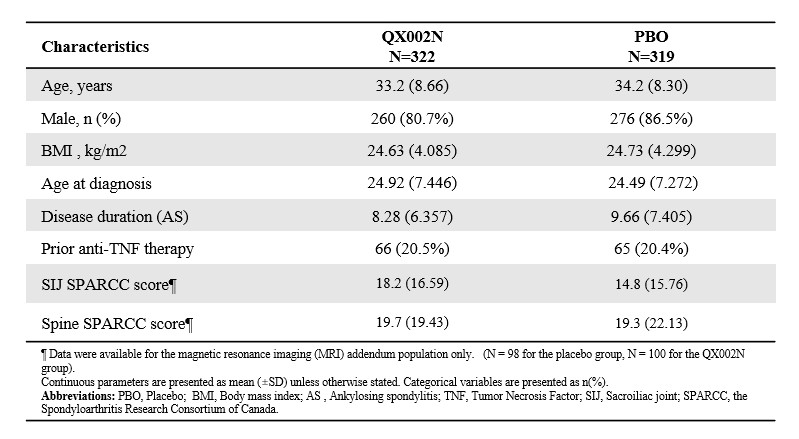 Table 1. Baseline characteristics.
Table 1. Baseline characteristics.
SPARCC SIJ and spine scores reported for only patients in MRI sub-study. MRI SPARCC SIJ and spine inflammation scores range from 0 to 72 and from 0 to 108, separately. Lower scores indicate less SIJ/spinal inflammation and negative changes represent improvements.
Abbreviations: *: p value ≤0.05; ***: p value ≤0.001; ****: p value ≤0.0001; 95%CI: 95% confidence intervals; δ: difference between treatment groups; AS, ankylosing spondylitis; ASAS: Assessment of Spondylarthritis international Society; ASAS40: ASAS 40% response; ASAS20: ASAS 20% response; ASAS5/6: ASAS 5/6 response; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; SIJ: Sacroiliac joint; SPARCC: the Spondyloarthritis Research Consortium of Canada; BASFI: Bath Ankylosing Spondylitis Function Index; BASMI: Bath Ankylosing Spondylitis Metrology Index; MASES: the Maastricht Ankylosing Spondylitis Enthesitis Score; ASQoL, Ankylosing Spondylitis Quality of Life; SD: standard deviation; LSM: Least Squares Means; SEM: Standard Error of the Mean.
To cite this abstract in AMA style:
Zeng X, Zhang S, LIU S, Li F, wang x, SUN L, Du H, Shi G, li y, zhang h, Zhang L, Wu J, zhou m, gu z, zhao y, fang m, Song Q, wang t. Effect of QX002N on Clinical and Radiographic Outcomes in Ankylosing Spondylitis: Results from a Phase III Randomized, Double-blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effect-of-qx002n-on-clinical-and-radiographic-outcomes-in-ankylosing-spondylitis-results-from-a-phase-iii-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-qx002n-on-clinical-and-radiographic-outcomes-in-ankylosing-spondylitis-results-from-a-phase-iii-randomized-double-blind-placebo-controlled-study/

.jpg)
.jpg)