Session Information
Session Type: Abstract Session
Session Time: 10:45AM-11:00AM
Background/Purpose: Cardiovascular disease is a major cause of morbidity and mortality in antiphospholipid syndrome (APS), with rates of accelerated atherosclerosis comparable to those seen in diabetes. Although the proatherogenic (and other) effects of IgG antiphospholipid antibodies (aPL) have been widely studied, emerging evidence suggests that IgA aPL—particularly IgA anti-β2-glycoprotein I (aβ2GPI)—may also contribute significantly to atherogenesis. For example, a population-based study demonstrated a fourfold increased risk of future atherosclerotic cardiovascular events among individuals with IgA aPL but no established autoimmune condition. In the same study, IgA aPL were associated with reduced cholesterol efflux capacity (CEC), a critical anti-atherosclerotic function of HDL. However, the mechanisms by which IgA aβ2GPI might promote vascular dysfunction and frank atherosclerosis remain poorly defined. Here, we aimed to investigate the role of IgA aβ2GPI in promoting atherosclerosis through effects on myeloid cells, HDL function, and vascular integrity.
Methods: Total IgA was purified from IgA aβ2GPI-positive patients (aβ2GPI IgA) and assessed in various in vitro and in vivo systems. Myeloid cell functional assays assessed CEC, fatty acid uptake, foam cell formation, and NETosis. Aortic endothelial function was tested in mice treated with either aβ2GPI IgA or control IgA. Vascular reactivity was measured by wire myography, and plasma HDL function was evaluated using a CEC assay.
Results: Compared with control IgA, treatment of macrophages with aβ2GPI IgA significantly enhanced fatty acid uptake and promoted foam cell formation—hallmarks of early atherogenesis. aβ2GPI IgA also impaired cholesterol efflux capacity, a critical anti-atherosclerotic function of HDL (Fig. 1). Furthermore, aβ2GPI IgA triggered robust NETosis by healthy primary neutrophils, an effect that was markedly reduced by blockade of the IgA receptor CD89 (Fig. 2). In vivo, mice treated with aβ2GPI IgA exhibited widespread vascular dysfunction. Wire myography demonstrated impaired acetylcholine-mediated relaxation of phenylephrine-contracted aortic rings (i.e., endothelium-dependent relaxation), consistent with endothelial impairment (Fig. 3A-B). Plasma from the same mice demonstrated significantly reduced cholesterol efflux capacity compared to controls, suggestive of HDL dysfunction (Fig. 3C). Collectively, these findings support a mechanistic model in which aβ2GPI IgA promotes proatherosclerotic vascular injury via three interrelated pathways: (1) induction of lipid-laden, pro-atherogenic macrophages; (2) CD89-dependent NETosis; and (3) systemic impairment of HDL function and endothelial responsiveness.
Conclusion: By driving foam cell formation, inducing CD89-mediated NETosis, and impairing HDL function and endothelial responsiveness, aβ2GPI IgA may have a role as a key mediator of atherogenesis in APS. Targeting IgA-driven mechanisms may offer new therapeutic avenues to mitigate cardiovascular risk in affected patients.
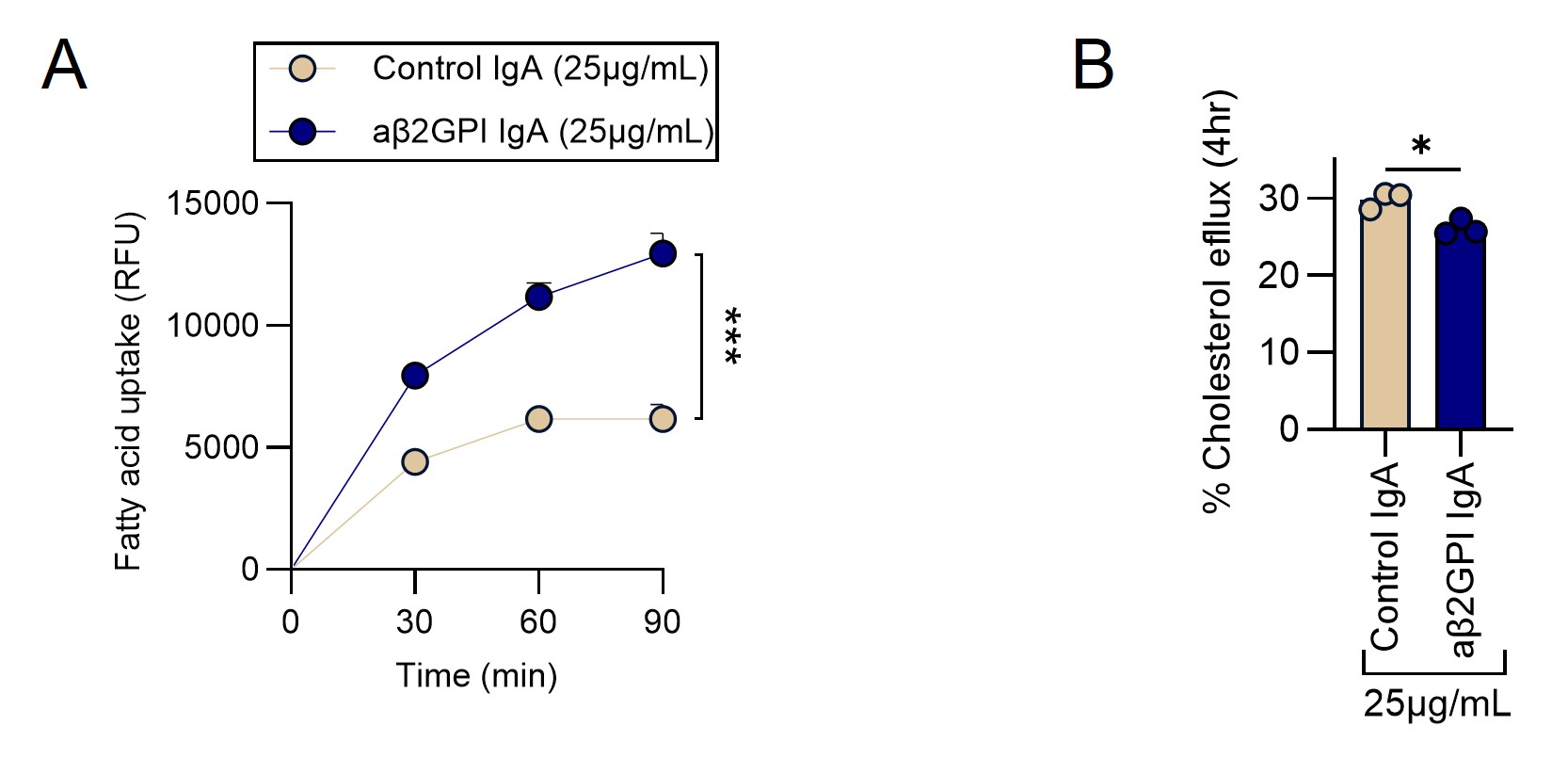 Figure 1: aβ2GPI IgA enhances fatty acid uptake in macrophages and impairs their cholesterol efflux capacity. (A) Macrophage fatty acid uptake was evaluated in real-time after 4hrs pre-treatment with 25 µg/mL of either control IgA or aβ2GPI IgA for 90 minutes. Fluorescent fatty acid analogs were used to assess intracellular uptake. (B) Comparison of % Cholesterol efflux at 4 hours after treatment with 25µg/mL of control or aβ2GPI IgA. Significance, *p < 0.05, and ***p < 0.001.
Figure 1: aβ2GPI IgA enhances fatty acid uptake in macrophages and impairs their cholesterol efflux capacity. (A) Macrophage fatty acid uptake was evaluated in real-time after 4hrs pre-treatment with 25 µg/mL of either control IgA or aβ2GPI IgA for 90 minutes. Fluorescent fatty acid analogs were used to assess intracellular uptake. (B) Comparison of % Cholesterol efflux at 4 hours after treatment with 25µg/mL of control or aβ2GPI IgA. Significance, *p < 0.05, and ***p < 0.001.
.jpg) Figure 2: aβ2GPI IgA induces NETosis in neutrophils from healthy donors. (A) Donor neutrophils were treated with aβ2GPI IgA (50 μg/mL) or control IgA (50 μg/mL). NETosis was quantified by SYTOX Green assay. (B) Neutrophils treated with IgA by microscopy (green=neutrophil elastase; red=myeloperoxidase; and blue=DNA; arrows indicate netting neutrophils). (C) Treatment with anti-CD89. Significance, **p < 0.01.
Figure 2: aβ2GPI IgA induces NETosis in neutrophils from healthy donors. (A) Donor neutrophils were treated with aβ2GPI IgA (50 μg/mL) or control IgA (50 μg/mL). NETosis was quantified by SYTOX Green assay. (B) Neutrophils treated with IgA by microscopy (green=neutrophil elastase; red=myeloperoxidase; and blue=DNA; arrows indicate netting neutrophils). (C) Treatment with anti-CD89. Significance, **p < 0.01.
.jpg) Figure 3: aβ2GPI IgA impairs global vascular function and promotes a proatherogenic vascular environment in a mouse model. (A) Schematic overview of the mouse model used to assess vascular dysfunction. (B) Ten-week-old mice were injected intravenously with control or aβ2GPI IgA (2mg/kg body weight) every three days over a 9-day period. On day 10, aortas were harvested, and vascular function was assessed via wire myography, measuring endothelium-dependent relaxation of aortic rings in response to acetylcholine. (C) Cholesterol efflux capacity was assessed in a macrophage cell line using 2% ApoB-depleted mouse plasma. *Significance: *p < 0.05.
Figure 3: aβ2GPI IgA impairs global vascular function and promotes a proatherogenic vascular environment in a mouse model. (A) Schematic overview of the mouse model used to assess vascular dysfunction. (B) Ten-week-old mice were injected intravenously with control or aβ2GPI IgA (2mg/kg body weight) every three days over a 9-day period. On day 10, aortas were harvested, and vascular function was assessed via wire myography, measuring endothelium-dependent relaxation of aortic rings in response to acetylcholine. (C) Cholesterol efflux capacity was assessed in a macrophage cell line using 2% ApoB-depleted mouse plasma. *Significance: *p < 0.05.
To cite this abstract in AMA style:
Sugur K, Liu C, Chong E, Yalavarthi S, Kmetova K, Liang W, Becker E, Poppei G, Sarosh C, Somanathapura N, Tambralli A, Knight J, Zuo Y. IgA Anti-β2GPI Antibodies Drive Proatherogenic Myeloid Activation and Vascular Dysfunction in APS [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/iga-anti-%ce%b22gpi-antibodies-drive-proatherogenic-myeloid-activation-and-vascular-dysfunction-in-aps/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/iga-anti-%ce%b22gpi-antibodies-drive-proatherogenic-myeloid-activation-and-vascular-dysfunction-in-aps/
