Session Information
Date: Sunday, October 26, 2025
Session Type: Abstract Session
Session Time: 3:00PM-3:15PM
Background/Purpose: The current 2024 ACR Lupus Nephritis guidelines recommend checking the urine protein to creatinine ratio (UPCR) every 6-12 months. Early recognition of lupus nephritis (LN), though, would lead to earlier introduction of treatment and better outcomes. A risk formula to project a patient’s risk of incident proteinuria within 10 years of SLE diagnosis would allow more frequent UPCR checks in the highest risk population.
Methods: The analysis is based on 1,418 members of the Hopkins Lupus Cohort who had been followed for longer than one year since SLE diagnosis. Subjects who developed proteinuria before or in the year of SLE diagnosis were excluded. A multivariate equation to predict the risk of incident proteinuria was developed using a forward-stepwise approach, considering each of the univariate associates in turn, and adding the variable that led to the greatest reduction in Akaike’s Information Criterion (AIC).
Results: Univariate associates of incident (new) proteinuria after 1 year from SLE diagnosis are shown in Table 1. Table 2 shows the multivariate predictors of incident proteinuria. Major risk factors included non-Caucasian race and anti-Sm. Moderate risk factors included male sex, anti-RNP, anti-dsDNA, and low C4 at the time of SLE diagnosis. Based on this model we estimated the 10-year risk as a function of the number of risk factors a patient has (Table 3). A person with no risk factors has only a 2.6% chance of developing proteinuria in 10 years, whereas a person with all 8 risk factors has a 49% chance.
Conclusion: We built a risk score for proteinuria in the next 10 years that would allow clinicians to identify a high risk subset of SLE patients to target for more frequent measurement of urine protein to creatinine AND to consider earlier renal biopsy at even low levels of proteinuria. The risk factors include well accepted demographic variables (non-Caucasian race, male sex) and serologic tests that are easily available (low C4, anti-Sm, anti-RNP and anti-dsDNA).
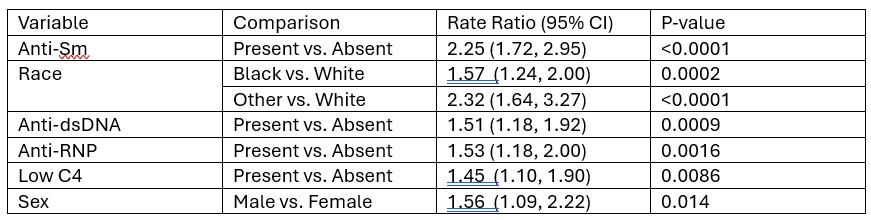 Table 1. Association between various manifestations in the first year after SLE diagnosis and eventual incidence proteinuria
Table 1. Association between various manifestations in the first year after SLE diagnosis and eventual incidence proteinuria
.jpg) Table 2. Multivariable model to predict incident proteinuria
Table 2. Multivariable model to predict incident proteinuria
.jpg) Table 3. Risk of incident proteinuria within 10 years of SLE diagnosis as a function of the number of risk factors (Each row in Table 2 counts as 1 risk factor except Anti-Sm and “Other” race which count as 2 risk factors)
Table 3. Risk of incident proteinuria within 10 years of SLE diagnosis as a function of the number of risk factors (Each row in Table 2 counts as 1 risk factor except Anti-Sm and “Other” race which count as 2 risk factors)
To cite this abstract in AMA style:
Petri M, Demirayak I, Fava A, Goldman D, Magder L. Risk of New Proteinuria in Next Ten Years in SLE [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-of-new-proteinuria-in-next-ten-years-in-sle/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-new-proteinuria-in-next-ten-years-in-sle/
