Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The diagnosis and management of giant cell arteritis (GCA) has significantly evolved over the last decades, mainly due to advances in imaging techniques and the introduction of new treatment modalities, respectively. Herein, we addressed the relapse rate, factors influencing relapses and the effect of introduction of interleukin-6-receptor targeting on relapse rates in GCA.
Methods: The REATS (GCA- Real-Life Assessment of Treatment Efficacy and Safety in GCA Patients) observational cohort included patients with a confirmed clinical diagnosis of GCA or a GCA flare between the first of January 2013 and 31st of December 2021. Six highly experienced German vasculitis centers participated and sequentially documented the disease course and treatment of GCA patients. Using a structured chart survey, we collected data on various outcomes, i.e. symptoms, treatments, acute-phase response, relapses. We used unsupervised clustering methods to identify symptom-based disease clusters and generalized additive models to analyze glucocorticoid dose and acute-phase response over time. Time-to-relapse was analyzed using survival methods.
Results: We included 395 patients with newly diagnosed GCA or a GCA flare. Diagnosis was supported by temporal artery ultrasound (37%), 18F-FDG-PET/CT (29%), temporal artery biopsy (14%) and by MRI (5,8%). The mean C-reactive protein value at disease onset or flare was 72,1 mg/dl, indicating a highly active cohort at the start of the documentation. The median total follow-up was 22.2 (CI 11.7-40.6) months. During this follow up time 97 of the 395 patients relapsed including 15 patients who relapsed more than once (Figure 1). The median (IQR) time to first relapse was 12.5 (CI 7.1-21.8) months. Most common symptoms at relapse were headache (31%) and PMR symptoms (20%) (Table 1). Though over 90 different baseline characteristics were analysed to identify risk factors for a future relapse, no significant predictors could be identified. None of the applied treatment regimes showed any superiority in preventing disease relapses. Looking at the relapse rates before (2013-2017) and after interleukin-6-receptor targeting (2017-2021) was introduced more relapses occurred after 2017 (Figure 2).
Conclusion: Relapses are frequent in GCA and did not decrease with introduction of interleukin-6-receptor targeting in GCA. No risk factor for relapses could be identified, despite many different risk factors were assessed. Also, none of the treatment regimens used appear to be superior in preventing relapses. Interestingly, since the introduction of interleukin-6-receptor targeting, relapse rate increased in GCA. This finding could be based on a less stringent use of pharmacotherapies in GCA, including glucocorticoids, considering that treatment options have become larger with the introduction of interleukin-6-receptor targeting.
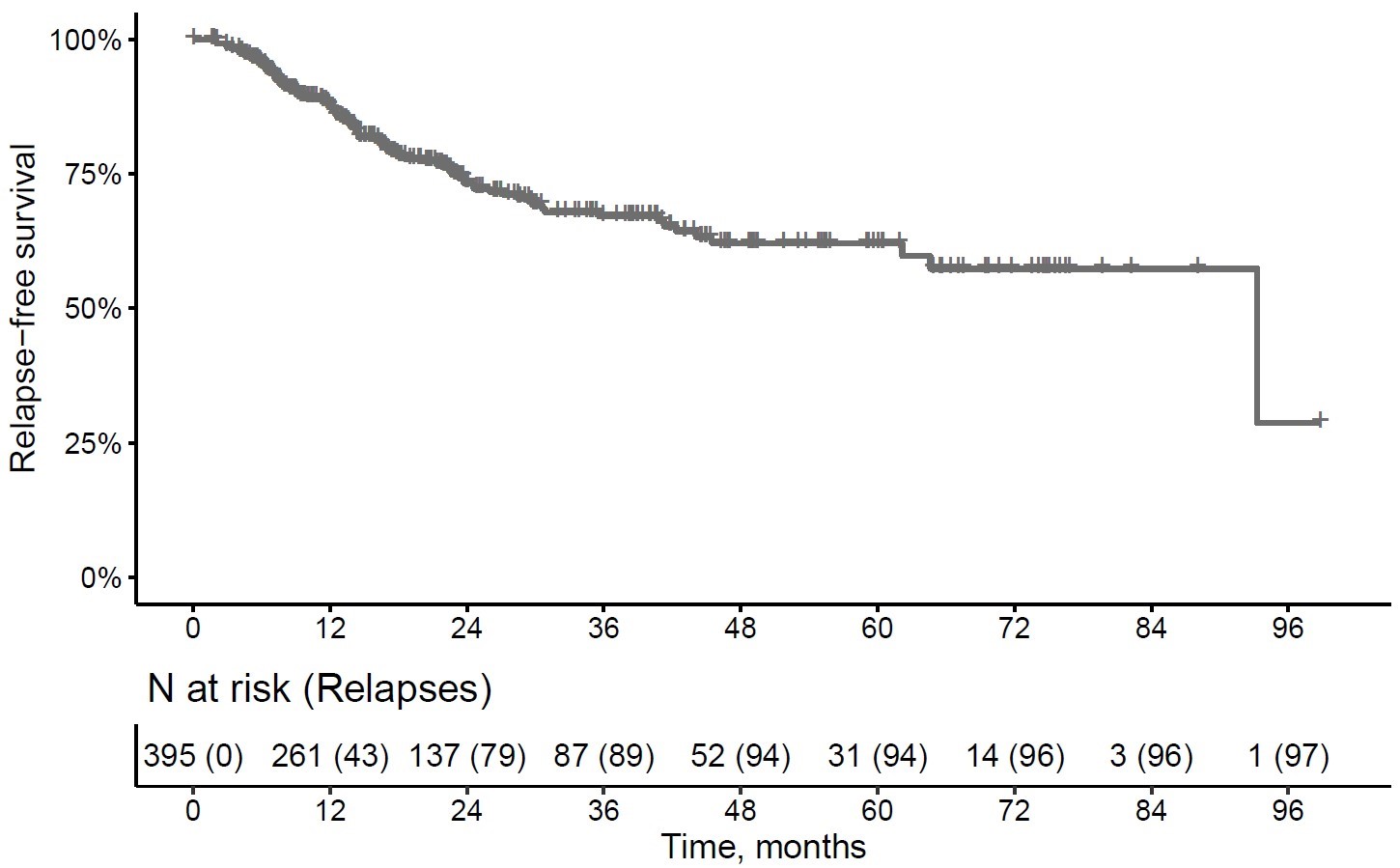 Figure 1 relapse-free survival
Figure 1 relapse-free survival
.jpg) Figure 2 relapse-free survival by era
Figure 2 relapse-free survival by era
To cite this abstract in AMA style:
Schoenau V, Corte G, Tascilar K, Hartmann F, Ott S, Schmidt W, Krause A, Alexander P, Oelzner P, Schmalzing M, Fröhlich M, Gernert M, Henes J, Venhoff N, Hellmich B, Manger B, Schett G, Rech J. Relapse rate, predictors of relapses and impact of introduction of interleukin-6-receptor inhibition on relapse rate in GCA- Data from the large REATS cohort from six vasculitis centers [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/relapse-rate-predictors-of-relapses-and-impact-of-introduction-of-interleukin-6-receptor-inhibition-on-relapse-rate-in-gca-data-from-the-large-reats-cohort-from-six-vasculitis-centers/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/relapse-rate-predictors-of-relapses-and-impact-of-introduction-of-interleukin-6-receptor-inhibition-on-relapse-rate-in-gca-data-from-the-large-reats-cohort-from-six-vasculitis-centers/

.jpg)