Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Aortitis associated to Giant cell arteritis (GCA) (GCA-aortitis) is one of the most severe manifestation of GCA. Tocilizumab (TCZ) has demonstrated efficacy in large-vessel vasculitis (LVV) including GCA, previous pharmacokinetic studies, have shown more stable and sustained levels of tocilizumab with the subcutaneous form compared to the intravenous form. However, there are no studies comparing intravenous (IV) vs subcutaneous (SC) administration of TCZ in GCA-aortitis.Our aim was to compare the effectiveness of TCZ in GCA-aortitis according the route of administration (IV vs SC) in a wide series of patients in a clinical world setting.
Methods: Multicentre study of 196 patients diagnosed with GCA-aortitis by imaging techniques and treated with TCZ. Patients were divided in two groups according to the route of administration of TCZ: i) IV, and ii) SC. GCA was diagnosed by 1990 ACR criteria, and/or temporal artery biopsy and/or imaging techniques. Aortitis was diagnosed mainly by 18F-FDG-PET/CT. Main outcomes were EULAR remission definition (clinical remission and normalization of CRP and ESR), imaging remission (considered when vascular FDG uptake was less than liver uptake in 18F-FDG PET/CT) and glucocorticoid withdrawal after TCZ initiation.
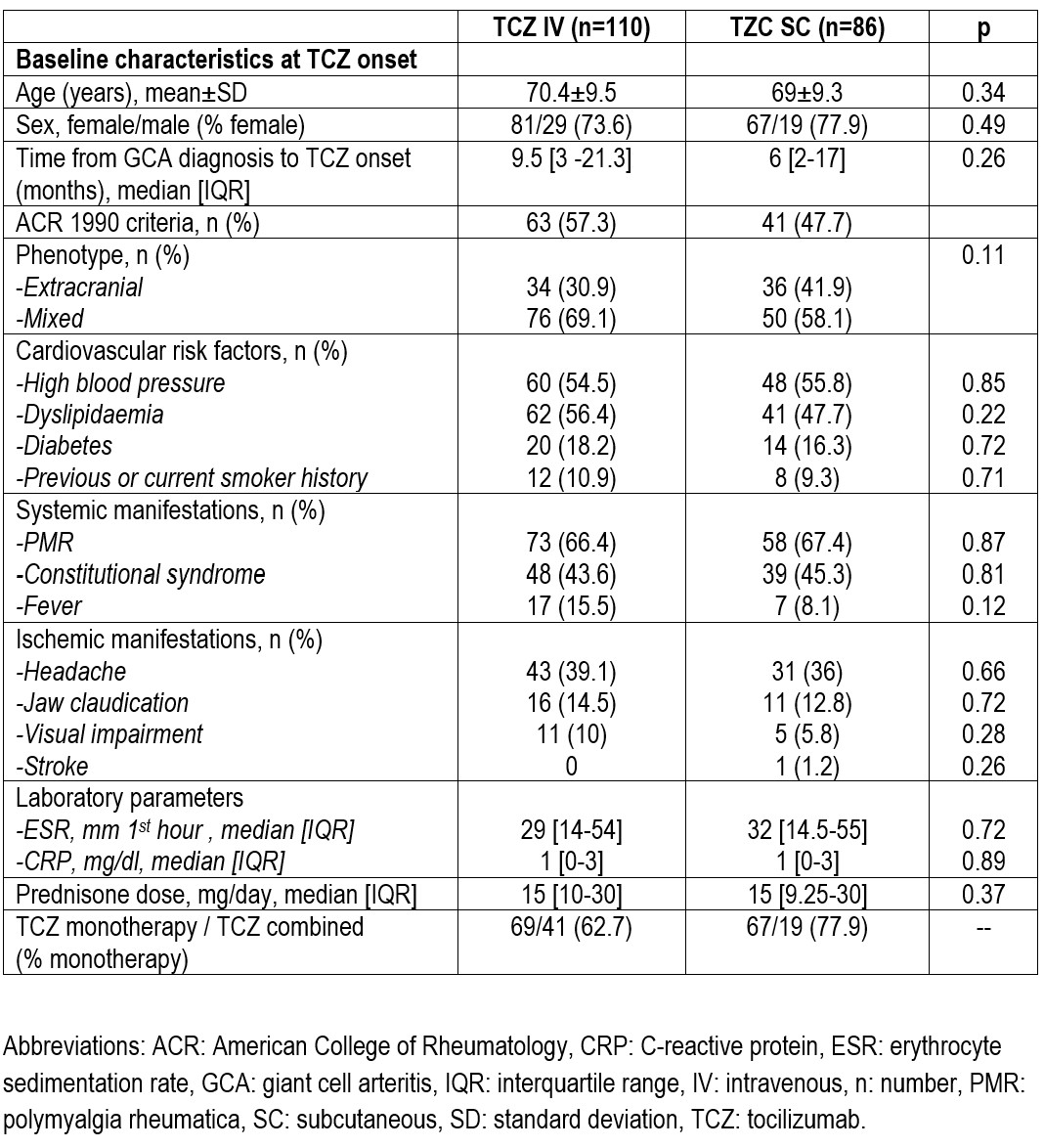
Results: We studied 196 patients (148 women 48 men) ,mean age 69.8±9.4 years treated with TCZ were included. One hundred and ten received IV-TCZ and 86 SC-TCZ (Table). Time between diagnosis of aortitis-GCA and TCZ initiation was shorter in SC group, although without reaching statistical significance. Interestingly, no differences in acute phase reactants were observed at TCZ initiation between both groups. There were no differences between the two routes of TCZ administration in glucocorticoid-sparing effect after TCZ initiation. Noticeably, statistical differences in EULAR remission definition were observed after 24-month follow-up, although there were no differences in imaging remission (Figure).
Conclusion: In GCA-aortitis, SC TCZ might be more effective than IV in achieving the EULAR remission target in clinical practice conditions after 24-month follow-up.
To cite this abstract in AMA style:
Secada-Gómez C, Loricera J, Martin-Gutierrez A, Castañeda S, Blanco R. Intravenous versus subcutaneous administration of Tocilizumab in aortitis associated with giant cell arteritis: multicenter open-label study of 196 patients [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/intravenous-versus-subcutaneous-administration-of-tocilizumab-in-aortitis-associated-with-giant-cell-arteritis-multicenter-open-label-study-of-196-patients/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intravenous-versus-subcutaneous-administration-of-tocilizumab-in-aortitis-associated-with-giant-cell-arteritis-multicenter-open-label-study-of-196-patients/

.jpg)