Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Background: Giant-cell arteritis(GCA) is a chronic inflammatory disease targeting large and medium-sized arteries. Inflammation-induced vascular remodeling leads to vascular occlusion, with ischemic complications. Myofibroblasts are key cells in this process, and they differentiate from quiescent vascular smooth muscle cells under the influx of several factors, including PDGFs produced by inflammatory cells, mainly macrophages(1). GM-CSF is a key proinflammatory cytokine with pleiotropic effects on a variety of cells. Macrophages, the predominant infiltrating cells in GCA, are among the main targets of GM-CSF. Using an ex-vivo culture of patient’s arteries in 3D matrix(2), we have previously shown that intimal myofibroblasts express GM-CSF receptors and that blocking GM-CSF receptor with mavrilimumab(MAV) inhibits key pro-inflammatory pathways(3). In a phase 2 clinical trial, MAV, along with a standardized prednisone taper, was superior to placebo in reducing the risk of GCA flare and increasing the rate of sustained remission at week 26(4), indicating that MAV efficiently blocks clinically-relevant inflammatory pathways in GCAPurpose: We aimed to investigate the impact of MAV on pathways involved in myofibroblast differentiation and vascular remodeling.
Methods: Three patients (1 placebo, 2 MAV) consented to have a second temporal artery biopsy (TAB) at the trial completion. Nineteen TAB from patients with GCA (obtained for diagnostic purposes) were cultured ex-vivo as published(2,3) and treated with placebo or MAV (20 mg/ml) for 5 days. Arteries were processed for RNA extraction, and candidate transcripts were assessed by RT-qPCR. The supernatants were processed for proteomic analysis using G-series Human cytokine array (640 proteins). Differences were analyzed by paired Wilcoxon test. GSEA, Hallmark, C2 curated gene sets, C3 motif gene sets, and C5 gene ontology gene sets were used for pathway analysis. We focused on pathways involved in myofibroblast differentiation and fibrosis.
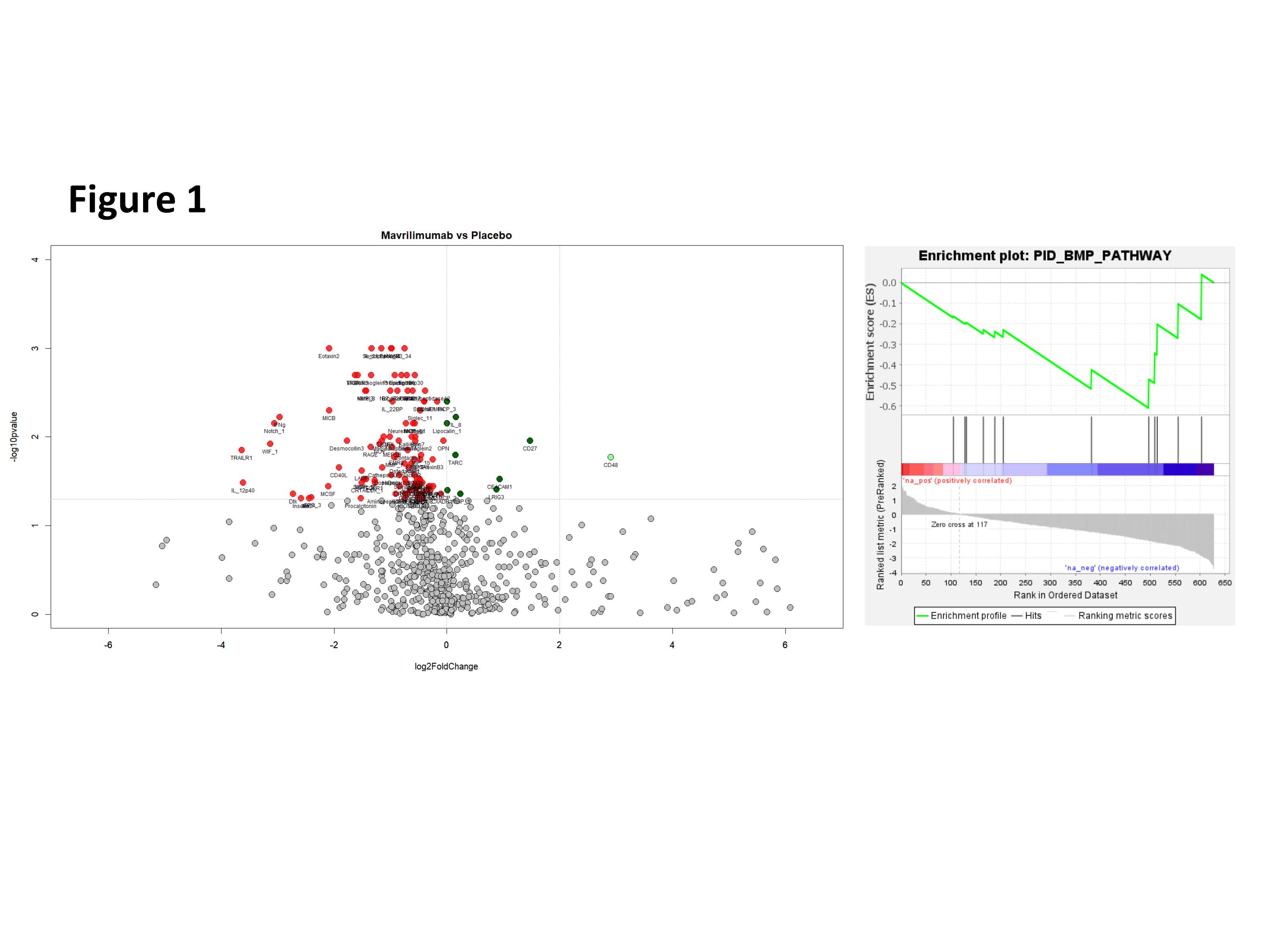
Results: Biopsies from MAV-treated patients showed better lumen preservation than the placebo-treated. MAV significantly reduced mRNA expression of PDGFA and B. No impact was seen in other relevant growth factors, such as FGF1, FGF2 or TGFb1 at the mRNA level. Pathway analysis of the proteins released into the supernatant revealed a significant impact of MAV on proteins related to BMP pathway (figure 1). A decrease in BMP-4(p=0.004), BAMBI (p= 0.07) and XIAP(p=0.053) was detected. In addition, a significant decrease in FGF12(p=0.0018) and FGF 17(p=0.0014) was also observed. A decrease in the corresponding mRNAs was also observed.
Conclusion: MAV modulates production of molecules involved in vascular remodeling in cultured arteries from patients with GCA. Whether MAV acts directly on myofibroblasts or indirectly through impairing macrophage function is being currently investigated. Further studies are ongoing to assess the functional impact of the modulated pathways on myofibroblast biology in GCA.
To cite this abstract in AMA style:
Corbera-bellalta m, Alba-Rovira R, Visocnik N, Kamberovic F, Araujo-Ayala F, Espigol-Frigolé G, Perez-Galán P, Bondensgaard K, Paolini J, Cid M. Blocking GM-CSF receptor alpha with mavrilimumab reduces production of growth factors involved in vascular remodeling in an ex-vivo model of temporal artery culture from patients with giant-cell arteritis (GCA) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/blocking-gm-csf-receptor-alpha-with-mavrilimumab-reduces-production-of-growth-factors-involved-in-vascular-remodeling-in-an-ex-vivo-model-of-temporal-artery-culture-from-patients-with-giant-cell-art/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/blocking-gm-csf-receptor-alpha-with-mavrilimumab-reduces-production-of-growth-factors-involved-in-vascular-remodeling-in-an-ex-vivo-model-of-temporal-artery-culture-from-patients-with-giant-cell-art/