Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Pain is one of the most debilitating symptoms of rheumatoid arthritis (RA) and a major contributor to reduced quality of life. Persistent joint pain severely limits daily activities and can lead to work disability, emotional distress, and diminished overall well-being. Olokizumab (OKZ) is a humanized anti-interleukin-6 monoclonal antibody approved for RA. We aimed to evaluate the impact of OKZ on patient-reported pain in RA through a meta-analysis of clinical and observational studies.
Methods: We systematically searched PubMed and eLibrary databases for studies reporting pain outcomes in RA patients treated with OKZ (Figure 1). Eligible sources included 3 randomized controlled trials (RCTs) and 4 real-world observational studies with pain assessments at 12 and 24 weeks. 3 RCTs and 2 observational studies were included for evaluation, while other 2 observational trials were currently withdrawn due to need in recalculation of certain measures of variance. A random-effects model (DerSimonian–Laird) was used to pool mean changes from baseline and mean differences versus placebo with corresponding 95% confidence intervals. When necessary, medians were converted to means and standard deviations based on Wan et al.’s method. All analyses were conducted in R (v4.4.1).
Results: In the RCTs, OKZ treatment led to clinically meaningful pain improvement compared to placebo. At 12 weeks, the mean reduction in patient pain (VAS) from baseline was greater in OKZ groups than in placebo by –14.05 points (95% CI –19.51 to –8.58) for 64 mg q2w and –13.89 (95% CI –19.52 to –8.56) for 64 mg q4w (both p < 0.001). These benefits were sustained or enhanced by Week 24, with OKZ-treated patients maintaining significantly greater pain reduction than placebo.Due to the non-comparative nature of observational studies they only revealed changes of pain in OKZ groups vs baseline. Real-world data echoed the findings in RCTs: patients on OKZ experienced robust pain relief over 12 weeks (figure 2), and the changes were sustained through week 24 when the mean pain reduction with OKZ reached -37.13 (-42.66 – 31.59) mm from baseline (Tyurin et al had data by 12 weeks only) (figure 3). All the changes vs baseline were significant (p < 0,001).
Conclusion: OKZ significantly reduces patient-reported pain in RA, with greater improvements than placebo observed in clinical trials and similarly robust pain relief reported in real-world studies. These meta-analytic findings underscore the value of OKZ as an effective therapy for managing pain in RA.
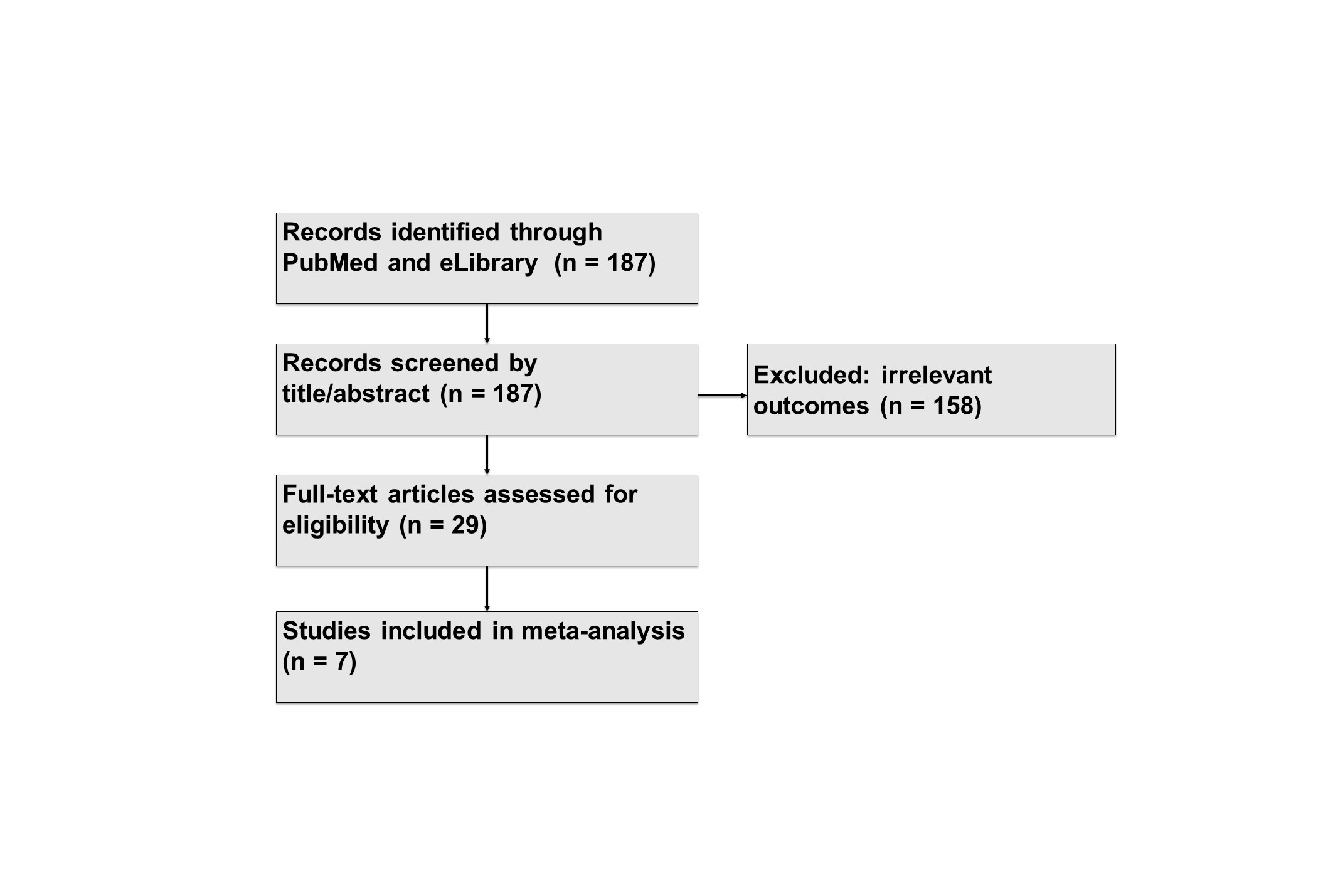 Figure 1. Literature search and study selection flowchart for pain outcomes
Figure 1. Literature search and study selection flowchart for pain outcomes
.jpg) Figure 2. Pooled mean change in pain (VAS 0–100) at Week 12 with OKZ 64 mg q4w.
Figure 2. Pooled mean change in pain (VAS 0–100) at Week 12 with OKZ 64 mg q4w.
CI- confidence interval
.jpg) Figure 3. Pooled mean change in pain at Week 24 with OKZ 64 mg q4w
Figure 3. Pooled mean change in pain at Week 24 with OKZ 64 mg q4w
CI- confidence interval
To cite this abstract in AMA style:
Karateev A, Kuzkina S, Egorova A, Polishchuk E. Impact of Olokizumab on Pain in Rheumatoid Arthritis: a Meta-analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-olokizumab-on-pain-in-rheumatoid-arthritis-a-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-olokizumab-on-pain-in-rheumatoid-arthritis-a-meta-analysis/
