Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In our previous Rheumatoid Arthritis Medication TAPering (RheumTAP) cohort study of patients with stable RA in remission on biologics +/- methotrexate at Allegheny Health Network (AHN), we found that the risk of flare was the lowest and insignificant with tapering methotrexate (MTX) alone (13%) compared to tapering biologics1. This result needed to be confirmed in larger studies given that current guideline recommendations on this topic are based on low level evidence. Thus, we built a collaborative cohort between AHN and Penn State Health (PSH) of patients with stable RA on MTX. A patient and provider survey within both cohorts demonstrated interest in MTX tapering2.
Methods: A retrospective cohort of adult patients with RA in sustained remission or low disease activity for at least 6 months while on MTX was created at both centers. The data were longitudinally followed between 1/1/2020-12/31/2022. The following tapering related variables were analyzed: reasons for MTX taper (remission, conception, infection, surgery, CKD, malignancy, side effect), tapering strategy (reduction vs. discontinuation), and tapering speed (gradual vs. rapid) along with others listed in Table 1. Clinical outcomes were compared between the groups at 3 and 6 months and the end of follow-up. Flare was defined as CDAI >10 and/or any worsening of disease activity leading to initiation of rescue therapy i.e. restoration of MTX dose or change of current therapy.
Results: Our cohort included a total of 100 patients with stable RA in remission or low disease activity while on MTX. Of these, 77 underwent MTX tapering. Baseline comparisons are listed in Table 1. MTX taper was initiated by the providers in 62 (80.5%) patients with the most common reason for tapering being remission (64%), followed by side effects/transaminitis (25%). Most patients had a MTX dose reduction (85.7%) vs stoppage (4.29%). Tapering was gradual (< 50% reduction over >4 weeks) in most patients. Median reduction in MTX dose was by 25% and 24 (31.58%) patients reduced MTX by at least 50%. There was no statistically significant difference in overall flare risk between MTX taper vs continuation groups at the end of follow-up period [n=34 (44.16%) vs 9 (37.5%), p=0.67] (Table 2). However, risk of flare was significantly higher in taper vs continuation group at 3 months [32 (41.55%) vs 3 (13.04%), RR 3.1, 95%CI 1.07-9.45, p=0.036]. There was no significant difference in proportion of patients in remission at 6 months [61 (79.2%) vs 13 (56.5%), RR 1.4, 95%CI 0.96 to 2.04, p=0.078]. In the taper group, most patients (88.24%) re-achieved remission with restoration of MTX dose.
Conclusion: In our retrospective cohort study on the outcomes of MTX tapering in well-controlled RA, patients who tapered or stopped MTX were no more likely to experience a flare by end of follow up period or sustain remission at 6 months after tapering than patients that continued MTX. However, the risk of flare after 3 months of MTX tapering was 3 times higher than MTX continuation which highlights the importance of close follow up after tapering. Most patients re-achieved remission with restoration of MTX after a flare.1. Tageldin et al, Rheumatology. 20232. Newman et al, Arthritis Rheumatol. 2024
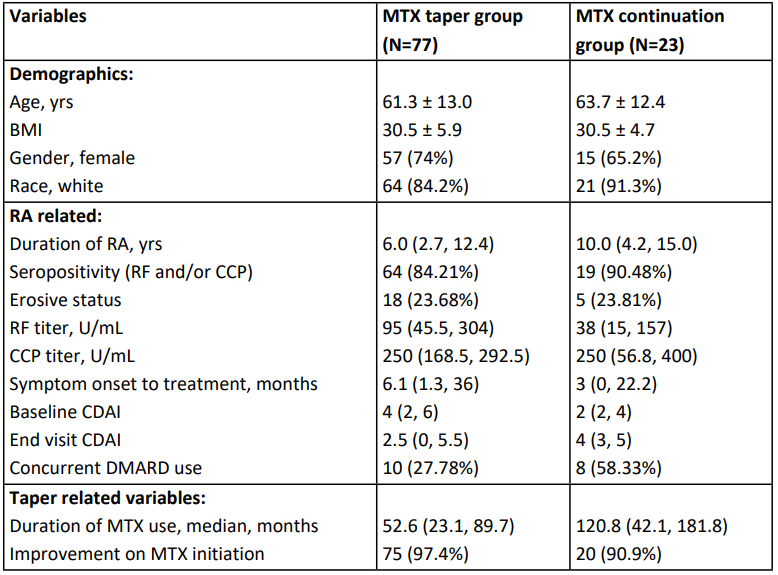 Comparison of MTX taper vs Continuation groups
Comparison of MTX taper vs Continuation groups
.jpg) Clinical Outcomes of MTX taper vs Continuation Groups
Clinical Outcomes of MTX taper vs Continuation Groups
To cite this abstract in AMA style:
Hajizadeh S, Sharma T, Newman P, Hernandez J, June R, Lehman E, Wilson N, Olsen N, Banks S. Is Methotrexate Tapering Possible In Stable RA? Clinical Observations of Methotrexate Tapering At Two Tertiary Care Centers [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/is-methotrexate-tapering-possible-in-stable-ra-clinical-observations-of-methotrexate-tapering-at-two-tertiary-care-centers/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-methotrexate-tapering-possible-in-stable-ra-clinical-observations-of-methotrexate-tapering-at-two-tertiary-care-centers/
