Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Peresolimab, a humanized IgG1 mAb, activates programmed cell death protein 1. In a phase 2a study, peresolimab demonstrated efficacy in participants (pts) with RA who had an inadequate response to prior DMARDs.1 Here, we present data from a phase 2b dose-ranging study of peresolimab.
Methods: This double-blind, placebo (PBO)-controlled study in adults with moderately to severely active RA (Nf490) randomized pts (1:2:2:2) to 100 mg (n=68), 400 mg (n=141), or 1000 mg (n=141) peresolimab or PBO (n=140) subcutaneously (SC) every 4 weeks (Q4W). At Week 12, pts receiving peresolimab continued their assigned dose; those receiving PBO switched (1:1) to 400 mg (n=67) or 1000 mg (n=73) peresolimab SC Q4W. The primary endpoint was 20% improvement in ACR response (ACR20) at Week 12; secondary endpoints included ACR50 and DAS28-CRP change from baseline (BL) at Week 12. Safety through 12 weeks and malignancies and possible major adverse cardiovascular events (MACE) through Week 60 are presented. A logistic regression model with treatment group, BL disease activity, and stratification factors as covariates was used for dichotomous endpoints. Missing data were imputed as non-response. A mixed-effects model for repeated measures with treatment group, BL disease activity, stratification factors, visit and treatment-by-visit interaction as fixed factors and pts as a random factor was used for continuous endpoints. Nominal p values are reported.
Results: At BL, the mean (SD) age was 54.0 (11.3) yrs, RA duration was 11.6 (8.2) yrs, and DAS28-CRP score was 5.97 (0.86); 92.4% were RF positive and 85.9% were anti-CCP positive, 33.5% were biological/target synthetic (b/ts) DMARD naïve, and 82.4% were female. A total of 30 (44.1%; p=0.534), 72 (51.1%; p=0.098), and 73 (51.8%; p=0.014) pts who received 100, 400, or 1000 mg peresolimab, respectively, achieved ACR20 at Week 12 vs 55 (39.3%) who received PBO (Figure 1a). Similarly, ACR50 at Week 12 was significantly different (p=0.003) in pts receiving 1000 mg peresolimab vs PBO but not for 400 mg (p=0.102) or 100 mg (p=0.741) peresolimab vs PBO (Figure 1b). The least squares mean (standard error) change from BL to Week 12 in DAS28-CRP was greater for pts receiving peresolimab 100 mg (-1.29 [0.15]), 400 mg (-1.41 [0.11]; p=0.030), and 1000 mg (-1.67 [0.11]; p < 0.001) vs PBO (-1.08 [0.11]). Treatment-emergent adverse events (AEs) were similar across groups through Week 12 other than 2 malignancies in each the 400 mg and 1000 mg peresolimab dose groups (Table 1). After Week 12, an additional 4 malignancies (non-melanoma skin cancer events excluded) were reported up to Week 60; 9 possible MACEs were reported in pts receiving peresolimab through Week 60.
Conclusion: Peresolimab was superior to PBO at Week 12 for several key endpoints, but efficacy was not differentiated from existing treatment options. In context of observed efficacy combined with the number of reported malignancies and possible MACEs through week 60, the study was stopped based on the non-favorable benefit/risk profile for peresolimab in treating RA patients.References:1. Tuttle, et al. N Engl J Med. 2023;388(20):1853–62
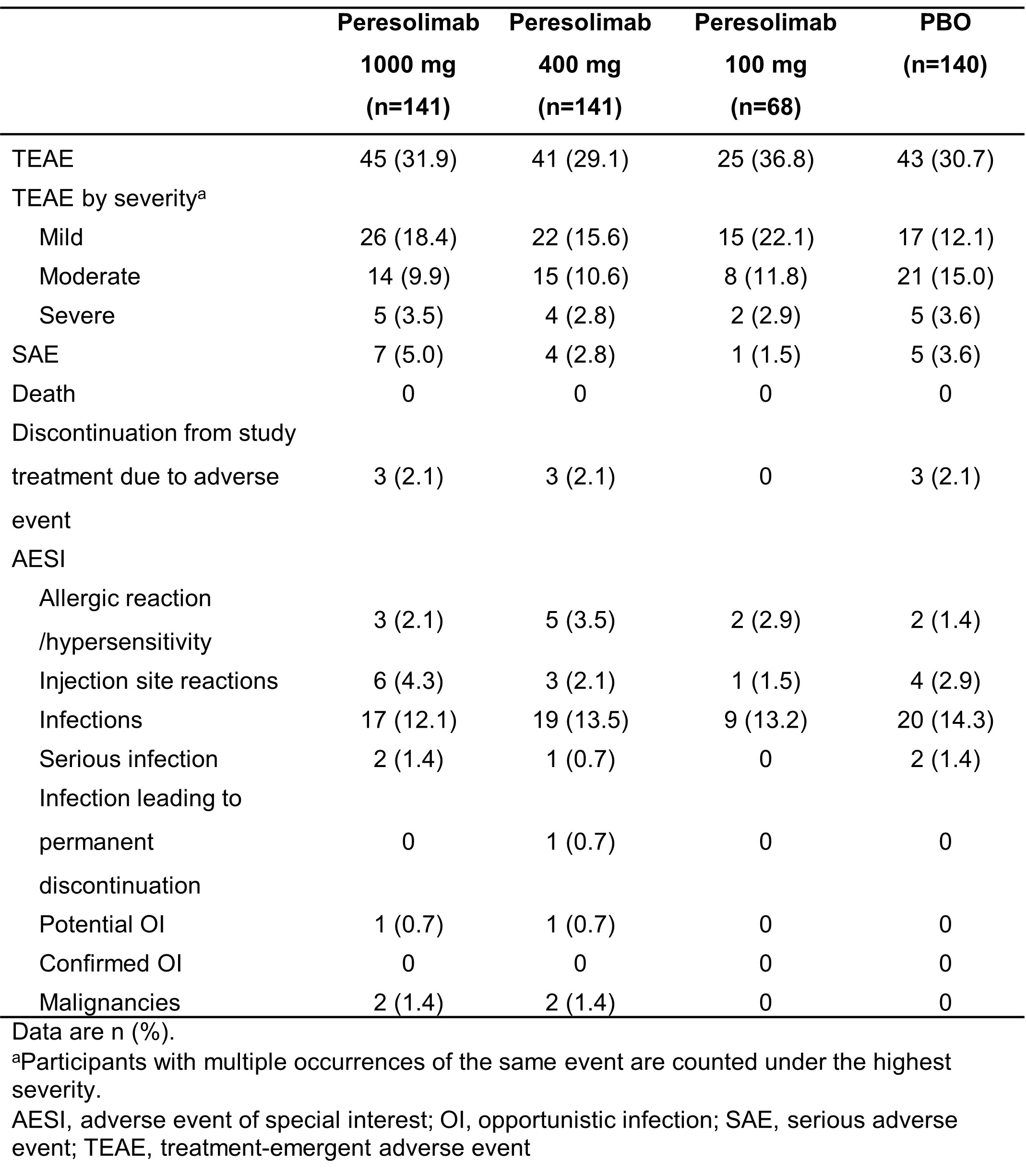 Figure 1. Proportion of participants with ACR20 and ACR50 response at Week 12.
Figure 1. Proportion of participants with ACR20 and ACR50 response at Week 12.
.jpg) Table 1. Overview of adverse events during Weeks 0 to 12
Table 1. Overview of adverse events during Weeks 0 to 12
To cite this abstract in AMA style:
Tuttle J, Griffing K, Genovese M, Rha H, Park S, Yachi P, Nirula A, PFEIFER L, Rayle T, Simón-Campos J, Bingham C, Winthrop K, Aletaha D, McInnes I, Xue Y, Tanaka Y, Fleischmann R, Emery P, Weinblatt M. A Phase 2b Dose-Ranging Study of Peresolimab for Adults with RA [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-phase-2b-dose-ranging-study-of-peresolimab-for-adults-with-ra/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2b-dose-ranging-study-of-peresolimab-for-adults-with-ra/
