Session Information
Date: Sunday, October 26, 2025
Title: (0430–0469) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients rate pain as one of the most important aspects of psoriatic arthritis (PsA). Pain signaling involves a series of cytokines, including those downstream of tyrosine kinase 2 (TYK2) inhibition. Deucravacitinib, an oral, selective allosteric inhibitor of TYK2, mediates signaling of key cytokines in PsA pathogenesis. Deucravacitinib was efficacious vs placebo in phase 3 trials in patients with active PsA. Deucravacitinib is approved for the treatment of adults with plaque psoriasis and is currently under investigation in two phase 3 trials in PsA (POETYK PsA-1 [NCT04908202] and POETYK PsA-2 [NCT04908189]). This analysis includes pooled data from both trials to characterize the effect of deucravacitinib on pain across 3 pain measures for the overall population and by sex to examine its relationship to pain intensity and differences in response to therapies.
Methods: Patients with PsA were randomized to receive deucravacitinib 6 mg (n = 648) or placebo (n = 646). All patients met the CASPAR classification criteria for PsA as part of study enrollment. Pain was assessed at baseline and Week 16 using the Patient Global Assessment (PGA) of Pain visual analog scale (VAS; scored from 0-100); Psoriatic Arthritis Impact of Disease (PsAID) pain instrument (scored from 0-10); and the 36-Item Short Form Survey (SF-36) Bodily Pain questionnaire (6-item Likert scale from “none” [0] to “very severe” [6]). Adjusted mean change in scores from baseline and proportion of patients with different levels of change to Week 16 were reported. Correlations among pain scales and disease activity were also evaluated.
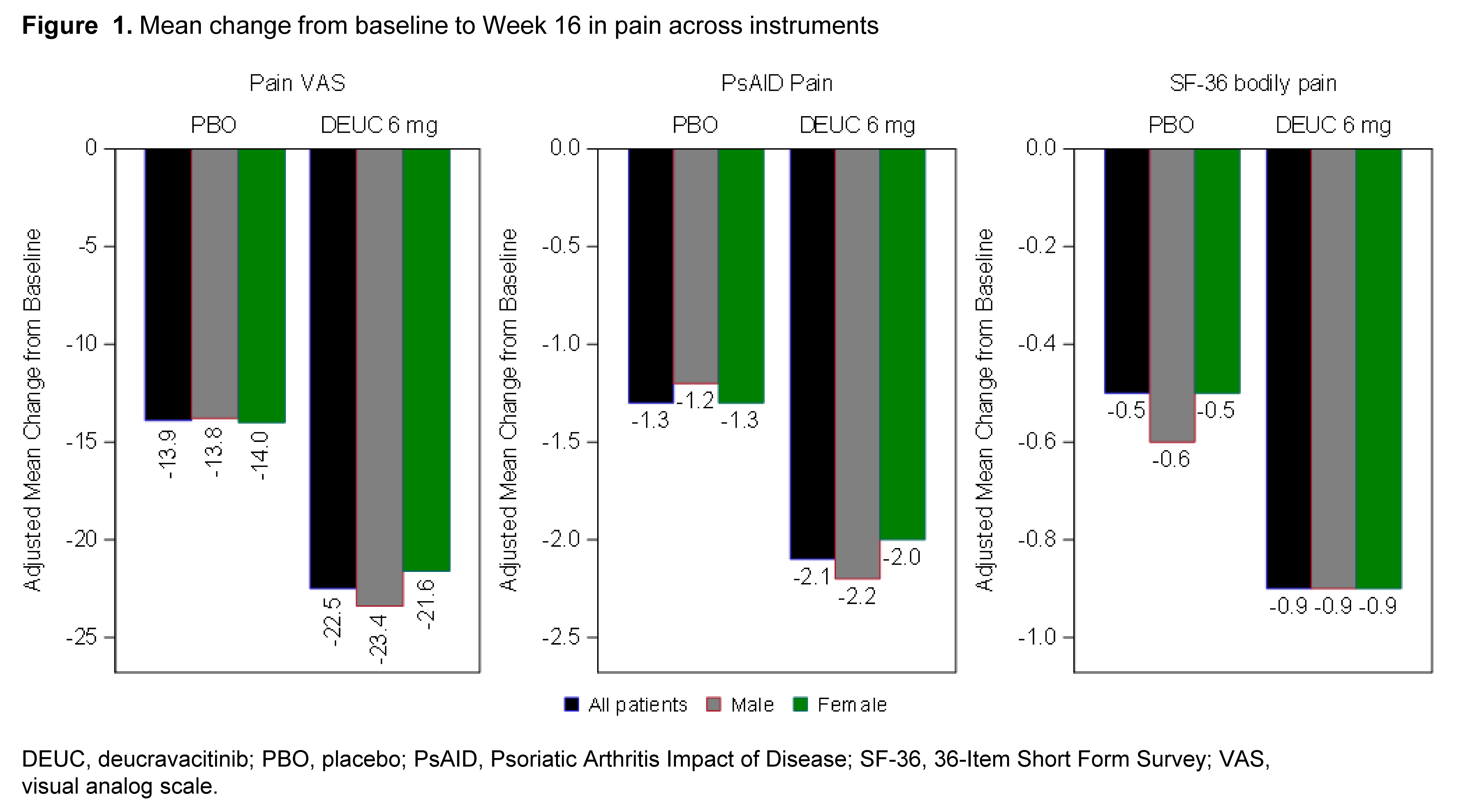
Results: At baseline, mean pain scores in the deucravacitinib vs placebo groups were: pain VAS 61.8 (20.45) vs 61.4 (20.68); PsAID Pain 6.3 (2.04) vs 6.3 (2.08); and SF-36 Bodily Pain 4.3 (0.96) vs 4.3 (0.98). All assessments of pain were strongly correlated with the disease activity at baseline. Overall, mean improvements in pain at Week 16 were greater in patients treated with deucravacitinib vs placebo across all measures. No consistent differences were observed between sexes in mean improvements in pain measures (Figure 1). In patients treated with deucravacitinib, nearly two-thirds reported improvements ≥ minimal clinically important difference, and >20% reported a 70% improvement in pain (Figure 2). Mean changes in patient-reported pain were highly correlated among pain instruments, demonstrating consistency (Figure 3).
Conclusion: Pooled data from 2 trials demonstrated a higher proportion of patients treated with deucravacitinib reported improvement in pain vs placebo, regardless of the instrument used. Improvements were generally consistent between males and females. These findings demonstrate that the use of deucravacitinib resulted in pain relief in patients with PsA.
To cite this abstract in AMA style:
Eder L, Mease P, Strand V, Ogdie A, Deodhar A, Haberman R, Armstrong A, Gottlieb A, Roberts D, Eliason L, Varga S, Vritzali E, Li J, Gossec L. Assessment of Pain Outcomes in Pooled Phase 3 Trials of a Selective, Tyrosine Kinase 2 Inhibitor, Deucravacitinib, in Patients With Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/assessment-of-pain-outcomes-in-pooled-phase-3-trials-of-a-selective-tyrosine-kinase-2-inhibitor-deucravacitinib-in-patients-with-active-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-pain-outcomes-in-pooled-phase-3-trials-of-a-selective-tyrosine-kinase-2-inhibitor-deucravacitinib-in-patients-with-active-psoriatic-arthritis/

.jpg)
.jpg)