Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Multisystem inflammatory syndrome in children (MIS-C) is a rare hyperinflammatory syndrome that follows SARS-CoV-2 infection. Prior plasma proteomic analysis from a 2020 cohort of MIS-C patients at our center revealed a profile characterized by macrophage-activation syndrome (MAS)-associated proteins, dysregulated interferon gamma responses and thrombotic microangiopathy. However, a limitation of that study, as well as most others at the time, was that samples were often acquired post-treatment. In this study, we interrogate the plasma proteome in untreated MIS-C patients versus pediatric patients with other viral syndromes in an independent cohort to identify signatures that uniquely define MIS-C unconfounded by treatment effects.
Methods: Plasma samples were collected from patients presenting to the Children’s Hospital of Philadelphia Emergency Department with fever upon their first blood draw and prior to any therapies. Samples were subsequently annotated based on final hospital diagnosis of MIS-C (Nf12) or viral syndrome (Nf30) as adjudicated by an expert panel. Analysis of 5400 plasma proteins was performed using the O-link Explore HT platform. Plasma auto-antibody analysis was performed using a customized Luminex auto-antibody array. Differential protein expression and pathway analysis were performed in R.
Results: Differential protein expression analysis confirmed that the MIS-C plasma proteome is distinct from that of patients with viral syndromes (Figure 1A) and pathway analysis revealed up-regulation of cytokine signaling pathways (Figure 1B). Unsupervised hierarchical clustering of differentially expressed proteins classified MIS-C from viral syndrome (Figure 1C). Consistent with findings in the 2020 cohort, sC5b9-associated proteins and MAS-associated proteins are more highly expressed and there is a disproportionally higher CXCL9 response to IFNg in MIS-C compared to viral infection (Figure 2A, B, C). Patients with MIS-C had lower expression of the IFNg suppressive protein TRIM21 in the 2020 cohort, however this was not reproduced in the current cohort (Figure 3A). Closer inspection revealed that only patients who received IVIg treatment prior to sampling had low TRIM21 expression in the 2020 cohort (Figure 3B). TRIM21 (also known as Ro52/SSA) is the antigen for anti-Ro52 autoantibodies. Plasma autoantibody profiling revealed anti-Ro52 antibodies in patients who had received IVIg (Figure 3C). Related anti-Ro60/SSA antibodies were low in samples collected prior to IVIg treatment and high in those sampled after IVIg (Figure 3D).
Conclusion: We have validated several unique features of the plasma proteome of MIS-C patients first identified in 2020. MIS-C plasma show high sC5b9 and MAS-associated protein expression and enhanced IFNg responsivity. Discrepant TRIM21 expression in these two cohorts is due to the presence of anti-Ro52 autoantibodies in IVIg-treated patients. These data support the use of plasma cytokine profiling and possibly a CXCL9/IFNg ratio to rapidly identify MIS-C patients. Furthermore, they highlight the presence of autoantibodies in IVIg and the need to interpret plasma proteomic data with caution in patients with prior IVIg exposure.
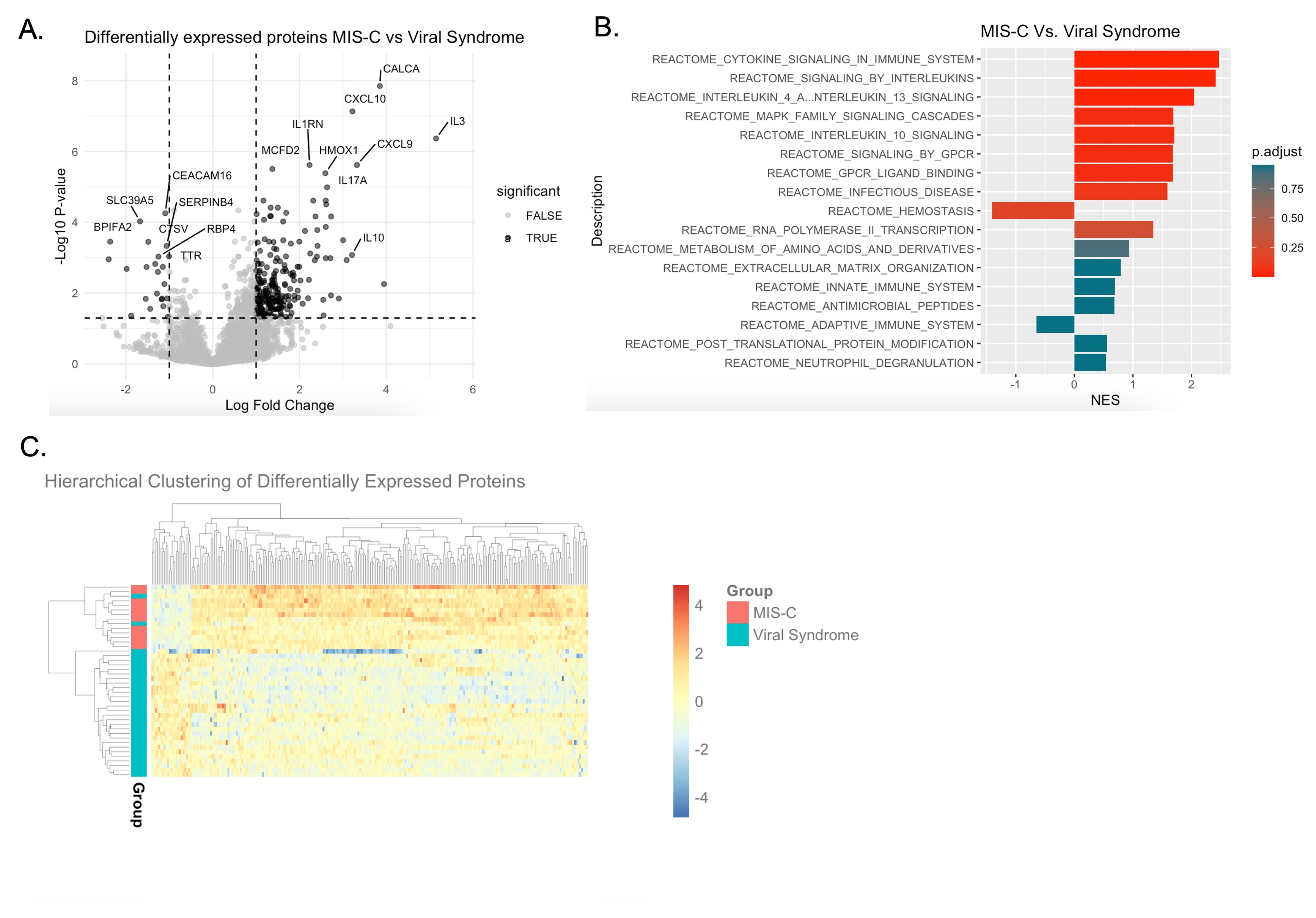 Figure 1 – The plasma proteome of MIS-C is distinct from other viral syndromes. (A) Differential protein expression analysis comparing plasma from patients with MIS-C (Nf12) and viral syndromes (Nf30). Differentially expressed proteins were considered significant when absolute log2 fold change was greater than 1 and Benjamini-Hochberg adjusted p-value less than 0.05. (B) Reactome pathway analysis of significantly differentially expressed proteins. (C) Hierarchical clustering of significantly differentially expressed proteins.
Figure 1 – The plasma proteome of MIS-C is distinct from other viral syndromes. (A) Differential protein expression analysis comparing plasma from patients with MIS-C (Nf12) and viral syndromes (Nf30). Differentially expressed proteins were considered significant when absolute log2 fold change was greater than 1 and Benjamini-Hochberg adjusted p-value less than 0.05. (B) Reactome pathway analysis of significantly differentially expressed proteins. (C) Hierarchical clustering of significantly differentially expressed proteins.
.jpg) Figure 2 – sC5b9 and macrophage activation syndrome (MAS)-associated plasma protein expression in MIS-C patients compared to patients with other viral syndromes. (A) Box and whisker plots of sC5b9-related proteins and NT-proBNP comparing MIS-C to other viral syndromes. p-values computed using Welsh’s T-test. Dots are individual patient normalized protein expression (NPX) values. (B) Box and whisker plots of MAS-associated proteins comparing MIS-C to other viral syndromes. p-values computed using Welsh’s T-test. Dots are individual patient NPX values. (C) Pearson correlation analysis between IFNg and CXCL9. Dots are individual patients colored by disease state. Colored areas represent 95% confidence intervals. ****p < 0.0001, ***p < 0.001, **p < 0.01.
Figure 2 – sC5b9 and macrophage activation syndrome (MAS)-associated plasma protein expression in MIS-C patients compared to patients with other viral syndromes. (A) Box and whisker plots of sC5b9-related proteins and NT-proBNP comparing MIS-C to other viral syndromes. p-values computed using Welsh’s T-test. Dots are individual patient normalized protein expression (NPX) values. (B) Box and whisker plots of MAS-associated proteins comparing MIS-C to other viral syndromes. p-values computed using Welsh’s T-test. Dots are individual patient NPX values. (C) Pearson correlation analysis between IFNg and CXCL9. Dots are individual patients colored by disease state. Colored areas represent 95% confidence intervals. ****p < 0.0001, ***p < 0.001, **p < 0.01.
.jpg) Figure 3 – TRIM21/Ro52 expression and anti-Ro52, anti-Ro60 autoantibody levels in MIS-C patients stratified by IVIg treatment status. (A) Box and whisker plots of TRIM21 comparing MIS-C to other viral syndromes. P-value computed using Welsh’s t-test. Dots are individual patient NPX values. (B) TRIM21 expression in MIS-C patients in 2020 cohort stratified by IVIg treatment status (untreated [pre-IVIg], treated [post-IVIg]). P-value computed using Welsh’s t-test. Dots are individual patient normalized protein expression (NPX) values. (C) Anti-Ro52 Ab MFI in MIS-C patients in 2020 cohort stratified by IVIg treatment status. Patients denoted by open triangles and open circles are the same patient in the TRIM21 and Ro52 Ab plot. (D) Anti-Ro60 Ab MFI in MIS-C patients in 2020 cohort stratified by IVIg treatment status (untreated [pre-IVIg], treated [post-IVIg]) and in replication cohort (all patients untreated [pre-IVIg]). *p < 0.05.
Figure 3 – TRIM21/Ro52 expression and anti-Ro52, anti-Ro60 autoantibody levels in MIS-C patients stratified by IVIg treatment status. (A) Box and whisker plots of TRIM21 comparing MIS-C to other viral syndromes. P-value computed using Welsh’s t-test. Dots are individual patient NPX values. (B) TRIM21 expression in MIS-C patients in 2020 cohort stratified by IVIg treatment status (untreated [pre-IVIg], treated [post-IVIg]). P-value computed using Welsh’s t-test. Dots are individual patient normalized protein expression (NPX) values. (C) Anti-Ro52 Ab MFI in MIS-C patients in 2020 cohort stratified by IVIg treatment status. Patients denoted by open triangles and open circles are the same patient in the TRIM21 and Ro52 Ab plot. (D) Anti-Ro60 Ab MFI in MIS-C patients in 2020 cohort stratified by IVIg treatment status (untreated [pre-IVIg], treated [post-IVIg]) and in replication cohort (all patients untreated [pre-IVIg]). *p < 0.05.
To cite this abstract in AMA style:
McCuaig S, Toland C, Konvinse K, Yang E, Utz P, Vella L, Odom John A, Bassiri H, Behrens E. Macrophage activation syndrome-associated proteins and enhanced interferon gamma responsiveness characterize the plasma proteome of patients with Multisystem Inflammatory Syndrome in Children (MIS-C) in a pre-treatment replication single center cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/macrophage-activation-syndrome-associated-proteins-and-enhanced-interferon-gamma-responsiveness-characterize-the-plasma-proteome-of-patients-with-multisystem-inflammatory-syndrome-in-children-mis-c/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/macrophage-activation-syndrome-associated-proteins-and-enhanced-interferon-gamma-responsiveness-characterize-the-plasma-proteome-of-patients-with-multisystem-inflammatory-syndrome-in-children-mis-c/
