Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: For children with limited juvenile idiopathic arthritis (JIA), defined as ≤4 affected joints and without uveitis, psoriasis, or sacroiliitis at presentation, it is unknown how often disease extends to polyarthritis or uveitis. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry is the largest prospective registry of children with JIA in North America, collecting longitudinal clinical and treatment data. The current study aimed to describe the natural history of limited JIA by estimating the proportion of recently diagnosed children who progress to develop polyarthritis, uveitis, or the need for systemic disease-modifying anti-rheumatic drugs (DMARDs), stratified by treatment status at the baseline Registry visit.
Methods: We conducted a retrospective analysis of data collected from the CARRA Registry between June 30, 2015 to July 16, 2021. Eligible children were 2-16 years at enrollment with a diagnosis of JIA within 6 months of enrollment and arthritis affecting ≤4 joints. Exclusion criteria included systemic JIA, sacroiliitis, inflammatory bowel disease, psoriasis, or uveitis. Patients were stratified based on systemic treatment status at the baseline Registry visit, defined as receipt of systemic DMARDs and/or systemic glucocorticoids for >14 days. The primary outcomes were incidence of disease extension within 12 and 18 months of enrollment, defined as the occurrence of polyarthritis (≥5 joints), uveitis, or need for systemic medications (e.g., conventional or biologic DMARDs or oral or intravenous glucocorticoids). We calculated the proportion of patients meeting each individual and the composite endpoint and conducted a Kaplan-Meier analysis.
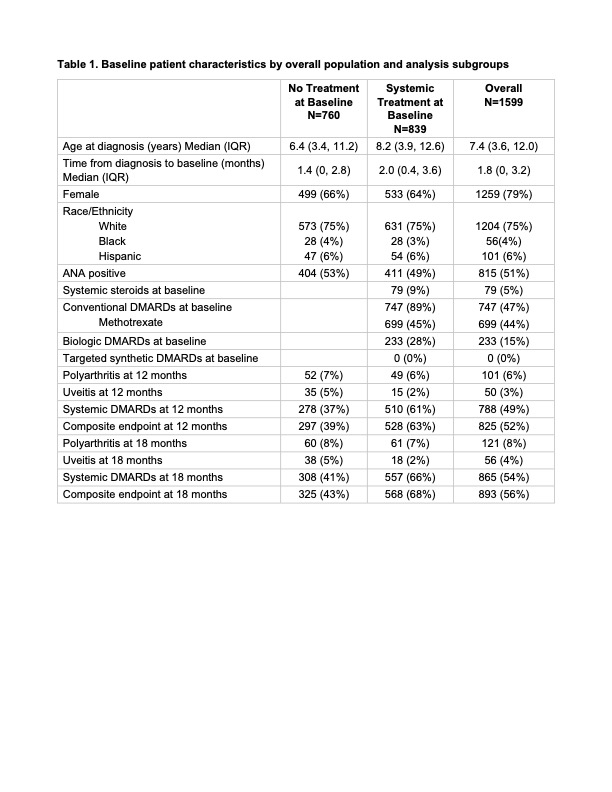
Results: A total of 1,599 children with limited JIA were included (Table 1). At enrollment, 53% were receiving systemic treatment, including systemic steroids (9%), methotrexate (83%), and biologic DMARDs (28%). Among children who were treatment-naïve at enrollment, 7% developed polyarthritis, 5% developed uveitis, and 37% initiated new systemic treatment within 12 months. By 18 months, 8% had developed polyarthritis, 5% uveitis, and 41% required new systemic treatment. The Kaplan-Meier estimate for the composite endpoint was 48% (Figure 1). Among those receiving systemic treatment at enrollment, 6% developed polyarthritis, 2% developed uveitis, and 61% were on systemic treatment at 12 months. At 18 months, 7% had developed polyarthritis, 2% uveitis, and 66% were on systemic treatment. The Kaplan-Meier estimate for the composite endpoint was 73% (Figure 1).
Conclusion: Among children with recently diagnosed limited JIA, nearly half received systemic DMARD within the first 6 months following disease diagnosis. Systemic medication use was not related to presence of polyarthritis or uveitis, suggesting clinicians are initiating systemic treatment early to manage persistent disease activity or prevent disease extension in limited JIA. Around 2 in 5 children who were treatment-naïve at Registry enrollment started systemic medication within 12 months. Over an 18-month period, the incidence of polyarthritis and uveitis was low across both treatment groups, with most extension occurring within the first 12 months.
To cite this abstract in AMA style:
Wu E, Balevic S, Marks A, Bhapkar M, Zhang B, Fist A, Kohlheim M, Del Gaizo V, Thomas L, Schanberg L. Disease Extension in Children with Limited Juvenile Arthritis in the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/disease-extension-in-children-with-limited-juvenile-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-extension-in-children-with-limited-juvenile-arthritis-in-the-childhood-arthritis-and-rheumatology-research-alliance-registry/

.jpg)