Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was formed by the Lupus Research Alliance as a public private partnership of people living with lupus, investigators, pharmaceutical companies, and the FDA to accelerate lupus drug development in a precompetitive, collaborative setting and ensure that the perspectives of people with lived experience of lupus are incorporated into the drug development process. We report on the first meeting to convene broad representation of lupus community voices to focus on better integration of patient-reported outcomes (PROs) in lupus clinical trials.
Methods: A public meeting with representatives from all Lupus ABC stakeholders was held October 16-17, 2024. The current use of PROs in lupus clinical trials was presented and breakout groups discussed 4 primary topics: 1) the important concepts of interest or PRO domains to measure; 2) questionnaire burden, modes of administration, and sharing results; 3) selection of reliable and valid PRO measures for trials; and 4) steps needed to advance the goal of wider PRO incorporation into trials. Results from breakout groups were presented and discussed; these are summarized below.
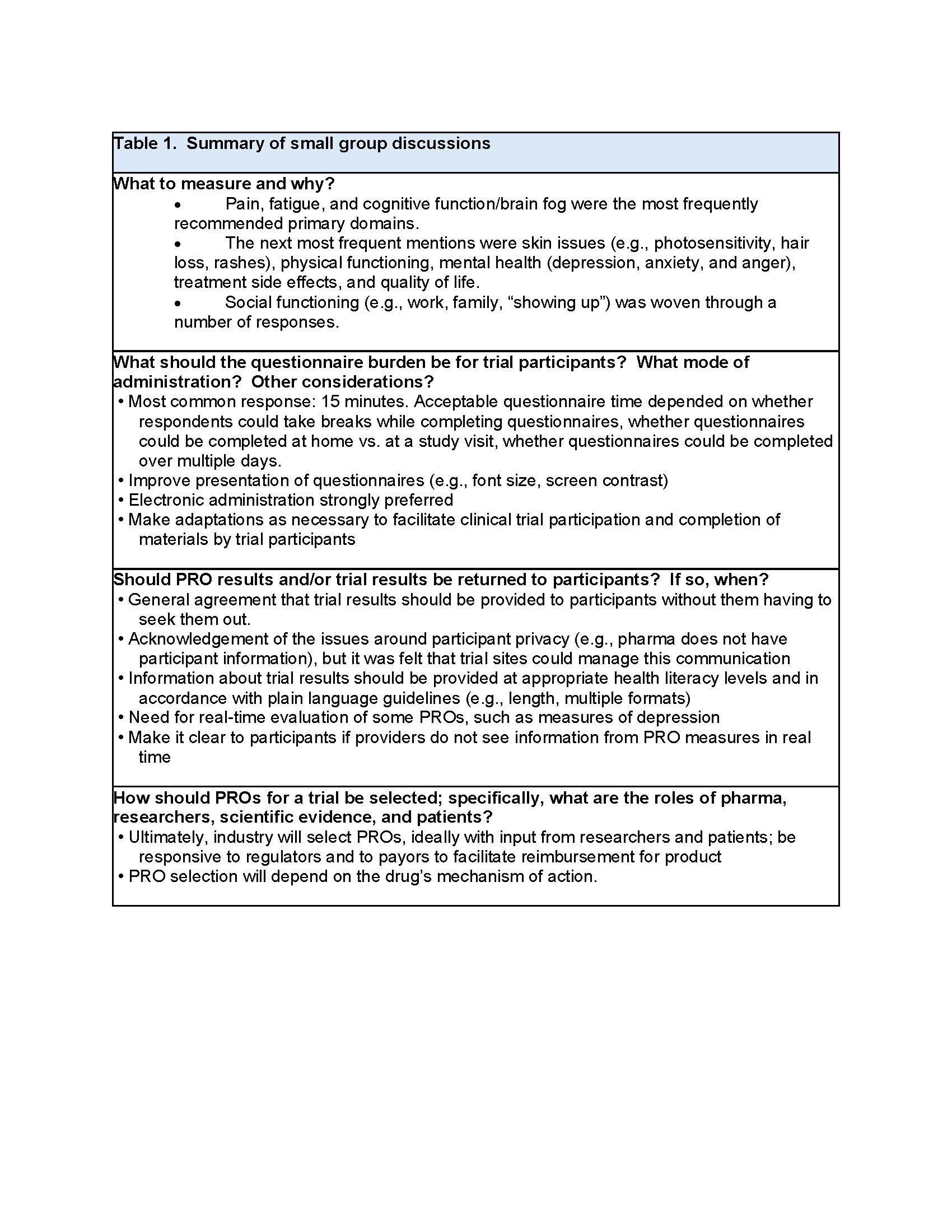
Results: Pain, fatigue, and cognitive function/brain fog were the most frequently recommended primary domains by participants with lupus and were corroborated with other stakeholders (Table 1). Ideas on questionnaire burden varied, and persons with lupus suggested potential adaptations of how PRO measures are currently administered depending on frequency, mode of administration and context, including a preference for electronic administration. There was strong consensus to return information on PROs and trial results to participants and involve all stakeholders early in the trial design process to identify the appropriate PRO domains and measures. While PROs are collected in most lupus clinical trials, participants felt that they may be underutilized as primary or secondary/exploratory endpoints. A desire to change the focus in trials from disease activity and signs to include PROs to highlight patients’ voices was noted. The need for balance between the mechanisms of action of a product, what is important to patients, and what aspect(s) of disease that could be changed from treatment was also discussed as a critical issue. The potential lack of concordance between standard clinical measures and PROs was felt to be a challenge to the acceptance of PROs as potential endpoints. The group noted specific steps that are needed to incorporate PROs into endpoints for regulatory decision-making: encourage PRO data to be made public and accessible to researchers; come to consensus on domain(s) and best measure(s) of those domains; and involve people living with lupus in selection of PRO domains and measures and trial design (Table 2).
Conclusion: The group, with broad lupus community representation, identified a number of steps needed to incorporate PRO measures into endpoints for regulatory decision-making. For these actions, collaborations among industry, researchers, regulators, payors, and people living with lupus are needed.
To cite this abstract in AMA style:
Katz P, Askanase A, Baruah N, Chen W, Cooper N, Fisch A, Garrard L, Jolly M, Vargas Lupo V, Menezes C, Mills J, Nguyen H, Staeva T, Park J, Touma Z. New efforts to incorporate patient-reported outcomes into clinical trials for lupus therapeutics [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/new-efforts-to-incorporate-patient-reported-outcomes-into-clinical-trials-for-lupus-therapeutics/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-efforts-to-incorporate-patient-reported-outcomes-into-clinical-trials-for-lupus-therapeutics/

.jpg)